Fig. 13.1
Artist illustration of the club-shaped surface projections (peplomers) that give coronaviruses their unique coronal fringe by negative-staining electron microscopy (Illustration by Adrian Galvin, New York, NY)
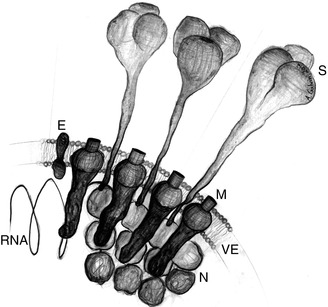
Fig. 13.2
Artist illustration of the major structural proteins and components common to all coronaviruses: S spike glycoprotein, M primary membrane glycoprotein, E envelope protein, N nucleocapsid protein enclosing the RNA helix, RNA RNA helix, VE viral envelope composed of host cell cytoplasm (Illustration by Adrian Galvin, New York, NY)
13.6 Immunology
SARS-CoV is internalized through binding of the spike glycoprotein to the host cell surface receptor angiotensin-converting enzyme 2 (ACE2) (Wang et al. 2004). Binding initiates conformational change in the spike that mediates fusion of the viral and host cell membranes and release of the nucleocapsid into the target cell allowing for disassembly and replication of the genome. Spike-mediated cell-to-cell fusion can occur and promotes syncytium formation and viral spread (Cheng et al. 2007).
Once internalized, the specific mechanism by which the human immune system responds to SARS-CoV is not well understood, and a particular area of controversy is the role of interferon (IFN). Cameron and colleagues measured plasma levels of IFN during the natural history of SARS in 40 patients. They found high IFN-alpha, IFN-gamma, and IFN-stimulated chemokine levels, and robust antiviral IFN-stimulated gene (ISG) expression was present early in the course of illness. Patients entered a crisis phase starting at approximately day 8, and most patients resolved IFN responses at crisis and expressed adaptive immune genes as they recovered. In contrast, patients with poor outcomes demonstrated deviated ISG and immunoglobulin gene expression levels, persistent chemokine levels, and deficient anti-SARS spike antibody production, suggesting a malfunction in the switch from innate to adaptive immunity (Cameron et al. 2007).
13.7 Clinical Features
The mean incubation period for SARS is 5 days with a range of 2–10 days. The clinical course of SARS follows a typical pattern that parallels viral load. The first week of illness is an influenza-like prodrome with fever, malaise, myalgia, headache, and rigors that coincide with increasing viral load. A decreasing viral load accompanies the second week of illness that is characterized by dry cough, dyspnea, and hypoxemia. Up to 70 % of patients develop large volume watery diarrhea. Clinical deterioration with rapidly progressive respiratory distress occurs in severe cases with approximately 20 % requiring intensive care. Progression to respiratory failure is the most common cause of death. Transmission occurs primarily in the second week (Hui and Chan 2010).
Chest radiographic and CT changes occur 3–4 days after onset of illness in most patients despite the lack of respiratory signs. Initial unilateral peripheral areas of ground glass and consolidation progress to multiple bilateral areas involving more than 80 % of lung characteristic of diffuse alveolar damage. In patients who survive the acute episode, traction bronchiectasis heralds the development of fibrosis and honeycomb lung (Fig. 13.3) (Chang et al. 2005).
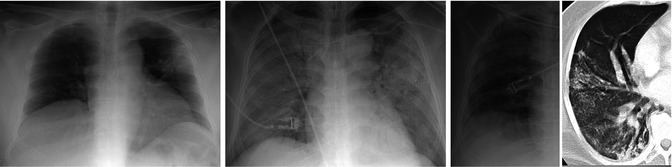

Fig. 13.3
Sequential chest radiographs in SARS patient. Anteroposterior portable chest radiograph (left) demonstrates focal consolidation in the left upper lobe. Anteroposterior portable chest radiograph (center) acquired 3 days later demonstrates consolidation of all five lobes with the patient intubated. Anteroposterior chest radiograph (right) acquired 3 months later demonstrates reticular opacities in the lung periphery. A chest CT acquired at the same time confirms the presence of fibrosis with traction bronchiectasis and reticular opacities
13.8 Pathologic Changes
SARS-CoV affects multiple organs but the major pathology is in the lungs. Diffuse alveolar damage (DAD) is the primary histologic finding, and the phase of DAD varies based on duration of illness. Cases of short duration, 10 days or less, demonstrate acute-phase DAD characterized by hyaline membranes lining alveolar walls, interstitial and airspace edema, mild chronic interstitial inflammation, and vascular congestion (Fig. 13.4). Bronchiolar injury is evidenced by luminal collections of fibrin associated with loss of cilia, denudation of bronchiolar epithelium, and deposition of fibrin on exposed basement membranes. Cases of more than 10 days duration exhibit organizing-phase DAD characterized by interstitial and airspace fibroblast proliferation accompanied by repair including type II pneumocyte hyperplasia and airway-centered squamous metaplasia. Hyperplastic type II cells show marked cytologic change, including cytomegaly, nucleomegaly, clearing of nuclear chromatin, and prominent nucleoli. Alveolar spaces contain a combination of macrophages and desquamated pneumocytes including multinucleated forms of both. Acute bronchopneumonia is a common feature in organizing-phase DAD, and fibrin thrombi may also be present. Intranuclear and intracytoplasmic inclusions have been variably reported, but SARS lacks a unique tissue response and cytopathic effect, making diagnosis by light microscopy alone difficult. After several weeks there can be progression of the organizing phase to the fibrotic phase, with extensive restructuring of the lung parenchyma and development of honeycomb lung (Franks et al. 2003).
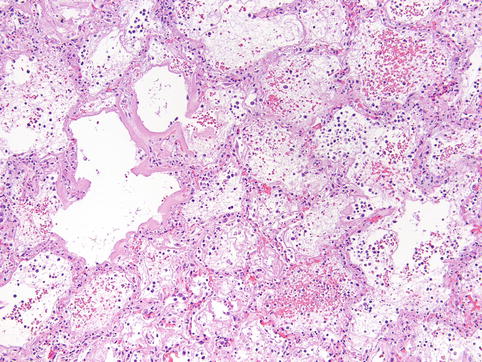

Fig. 13.4
Acute-phase DAD in SARS patient. Acute-phase DAD is characterized by eosinophilic hyaline membranes plastered against alveolar walls, interstitial and airspace edema, and mild chronic interstitial inflammation (100×, hematoxylin-eosin stain)
13.9 Diagnosis
There are no clinical or laboratory findings that reliably diagnose SARS-CoV infection early or rapidly enough to inform management decisions that must be made soon after a patient enters the healthcare system in order to contain potential infection. The Centers for Disease Control and Prevention (CDC) recommends that the diagnosis of SARS-CoV infection and initiation of isolation and stringent infection control measures should be based on risk of exposure. In the absence of person-to-person transmission of SARS-CoV anywhere in the world, the diagnosis of SARS-CoV infection should be considered only in patients who require hospitalization for radiologically confirmed pneumonia and who have an epidemiologic history that raises suspicion of SARS-CoV infection. Suspicion is heightened when the patient, within 10 days of onset of illness, has a history of recent travel to mainland China, Hong Kong, or Taiwan, or close contact with ill persons with a history of travel to these areas, or is employed in an occupation at risk for SARS-CoV, or is part of a cluster of cases of atypical pneumonia without an alternative diagnosis. Laboratory testing for SARS-CoV is available, including antibody detection by enzyme immunoassay (EIA) and reverse transcription polymerase chain reaction (RT-PCR). However, the positive predictive value of a diagnostic test is very low in the absence of person-to-person transmission worldwide, and the CDC recommends testing be performed judiciously and in consultation with local or state health departments (CDC 2005).
13.10 Differential Diagnosis
Initial signs and symptoms of SARS are nonspecific and common, which generates a wide differential diagnosis of respiratory pathogens including influenza virus, parainfluenza viruses, respiratory syncytial virus, Haemophilus influenza, Mycoplasma pneumonia, Chlamydia species, Legionella species, Coxiella burnetii, and other human coronaviruses (WHO 2004b).
13.11 Prevention
At the time of this writing in October 2013, the world is in an interepidemic period for SARS. The greatest risk of recurrence is from emergence or introduction of SARS-CoV from laboratories and emergence of SARS-CoV-like viruses from wildlife or other animal reservoirs. If SARS recurs, early detection of infected individuals is essential to contain local spread of infection and prevent international dissemination. Primary responsibility for risk assessment and management of SARS is with national health authorities, for example, the CDC in the United States. However, in its role coordinating global and regional surveillance, the WHO has revised its guidelines for global surveillance and reporting of SARS and has provided a framework of activities at national and international levels for risk assessment of SARS (WHO 2004b, c).
13.12 Treatment and Outcome
Many drugs were empirically tried during the epidemic, but no treatment has been shown to consistently improve the outcome of SARS patients, and supportive medical care remains the primary therapy. The case fatality ratio for SARS ranges from 0 % to more than 50 % depending on age group, with an overall estimate of 11 %. Case fatality is estimated to be less than 1 % for people aged 24 years and younger, 6 % for 25–44 years, 15 % for 45–64 years, and over 50 % for people aged 65 years and older (WHO 2003b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


