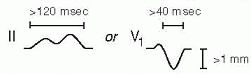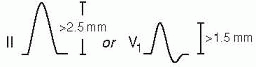Rate (? tachy or brady)
Rhythm (? P waves, ? relationship between P and QRS, ? regular)
QRST changes (? Q waves, poor R-wave progression V1-V6, ST ↑/↓ or T-wave Δs)
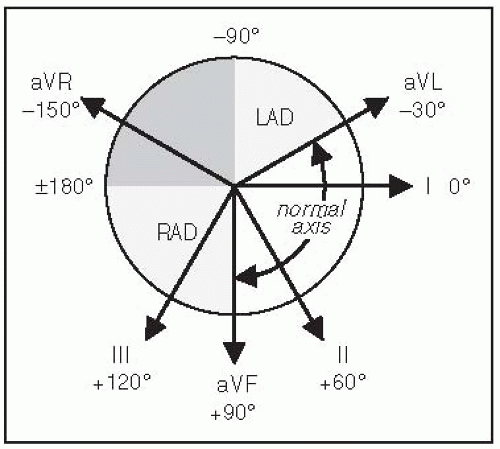
Definition: axis beyond -30° (S > R in lead II)
Definition: axis beyond +90° (S > R in lead I)
| |||||||||||||||
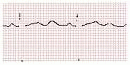
QT measured from beginning of QRS complex to end of T wave (measure longest QT)
QT varies w/ HR → corrected w/ Bazett formula: QTc = QT/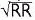 (RR in sec, can be estimated by 60/HR), overcorrects at high HR and undercorrects at low HR (nl QTc <440 msec ♂ and <460 msec ♀)
(RR in sec, can be estimated by 60/HR), overcorrects at high HR and undercorrects at low HR (nl QTc <440 msec ♂ and <460 msec ♀)
Fridericia’s formula preferred at very high or low HR: QTc = QT/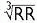
QT prolongation a/w ↑ risk TdP (espec >500 msec); perform baseline/serial ECGs if using QT prolonging meds, no estab guidelines for stopping Rx if QT prolongs
Etiologies:
Antiarrhythmics: class Ia (procainamide, disopyramide), class III (amio, sotalol, dofet)
Antimicrobials: macrolides, quinolones, azoles, pentamidine, atovaquone, atazanavir
Other: antiemetics (droperidol, 5-HT3 antagonists), alfuzosin, methadone, ranolazine
Electrolyte disturbances: hypoCa (nb, hyperCa a/w ↑ QT), ± hypoK, ? hypoMg
Congenital (long QT syndrome): K, Na, & Ca channelopathies (Circ 2013;127:126)
Romhilt-Estes point-score system (4 points = probable; 5 points = diagnostic):
Criteria
Points
Voltage (any of the following): R or S in limb leads ≥20 mm; S in V1 or V2 ≥30 mm; R in V5 or V6 ≥30 mm
3
ST-T displacement opposite to QRS deflection (either):
3
1
Left atrial enlargement
3
Left axis deviation
2
QRS duration ≥90 msec
1
Delayed intrinsicoid deflection (QRS onset to R peak) in V5 or V6 >50 msec
1
Sokolow-Lyon: S in V1 + R in V5 or V6 ≥35 mm or R in aVL ≥11 mm
Other: dextroversion; Duchenne muscular dystrophy; lead misplacement; nl variant
Definition: ≥30 msec (≥20 msec V2-V3) or >25% height of R wave in that QRS complex
Coronary spasm (Prinzmetal’s angina; transient STE in a coronary distribution)
HCM, Takotsubo CMP, ventricular aneurysm, cardiac contusion
Repolarization abnormalities
LBBB (↑ QRS duration, STE discordant from QRS complex) dx of MI in setting of LBBB: Sgarbossa criteria (NEJM 1996;334:481) ≥1 mm STE concordant w/ QRS (Se 73%, Sp 92%) STD ≥1 mm V1-V3 (Se 25%, Sp 96%) STE ≥5 mm discordant w/ QRS (Se 31%, Sp 92%)
LVH (↑ QRS amplitude)
Hyperkalemia (see below)
Hypothermia: Osborn waves
Early repolarization: most often seen in V2-V5 in young adults (JACC 2015;66:470)
1-4 mm elev of peak of notch or start of slurred downstroke of R wave (ie, J point); ± up concavity of ST & large Tw ( ratio of STE/T wave <25%; may disappear w/ exercise)
ratio of STE/T wave <25%; may disappear w/ exercise)
Hypokalemia (± U wave)
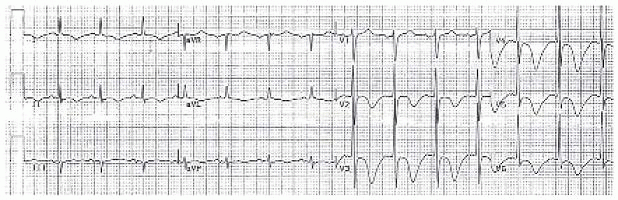 (Wellens’ sign, from Cuculich PS and Kates AM. The Washington Manual Cardiology Subspecialty Consult, 3rd ed. Philadelphia: Wolters Kluwer Health, 2014:286.)
(Wellens’ sign, from Cuculich PS and Kates AM. The Washington Manual Cardiology Subspecialty Consult, 3rd ed. Philadelphia: Wolters Kluwer Health, 2014:286.)
Posttachycardia or postpacing
Electrolyte, digoxin, PaO2, PaCO2, pH or core temperature disturbances
Intracranial bleed (“cerebral T waves,” usually w/ ↑ QT)
Normal variant in children (V1-V4) and leads in which QRS complex predominantly [circled dash]
QRS amplitude (R + S) <5 mm in all limb leads & <10 mm in all precordial leads
| ||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
Focused history: quality & severity of pain; location & radiation; provoking & palliating factors; intensity at onset; duration, frequency & pattern; setting in which it occurred; associated sx; cardiac hx and risk factors
Pain Feature
Classic for ACS
Atypical for ACS
Quality
Pressure (⊕ LR 1.3), tightness, “Levine” sign (clenched fist over chest), squeezing, fullness, heavy weight, more than prior angina or ≈ prior MI (⊕ LR 1.8)
Sharp (⊕ LR 0.3)
Region/radiation
Substernal, radiating to L or R arm or shoulder (⊕ LR 2.3-4.7), jaw, teeth, neck
Small area, points w/ 1 finger, radiation to back
Provocation
Exertion (⊕ LR 2.4; but may be absent)
Associated sx
(JAMA 2005;294:2623)
Targeted exam: VS (including BP in both arms), cardiac gallops, murmurs or rubs; signs of vascular disease (carotid or femoral bruits, ↓ pulses), signs of heart failure; lung & abdominal exam; chest wall exam for reproducibility of pain
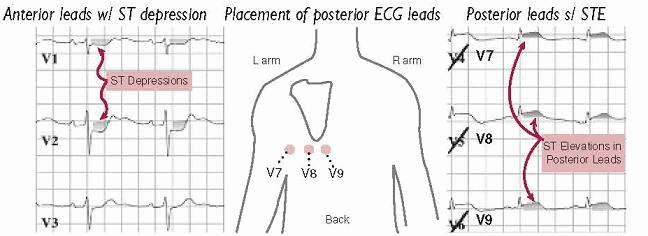
posterior leads (V7-V9; remove V4-V6 and place in post axillary, mid-clavic & L paraspinal position) useful to [check mark] for posterior STEMI if hx c/w ACS but stnd ECG unrevealing, espec if ST ↓ V1-V4 (ant ischemia vs post STEMI) or R/S V1-V2 > 1
R-sided leads (place V3-V6 in mirror image position on R side of chest) in inferior STEMI to detect RV involvement
Troponin: level >99th %ile w/ rise & fall in approp. setting is dx of MI; >95% Se, 90% Sp Detectable 1-6 h after injury, peaks 24 h, may remain elevated for 7-14 d in STEMI Tn may be ↑ in CKD in absence of ACS, add CK-MB/serial Tn for confirmation Sensitive Tn assays: 98% Se, 90% Sp, 75% PPV, 99% NPV w/in 3 h of admit to ED, 82% Se & 95% NPV at time of admission to ED (JAMA 2011;306:2684)
add CK-MB/serial Tn for confirmation Sensitive Tn assays: 98% Se, 90% Sp, 75% PPV, 99% NPV w/in 3 h of admit to ED, 82% Se & 95% NPV at time of admission to ED (JAMA 2011;306:2684)
High-sensitivity Tn assays quantify Tn in majority of healthy individ.; prog value in asx general pop., stable CAD & DM (NEJM 2009;361:2538 & 2015;373:610; JAMA 2010;304:2503)
Sources include skeletal muscle, tongue, diaphragm, intestine, uterus, prostate
CK-MB relative index (ratio of CK-MB to CK) >2.5-3 suggests cardiac vs skel. muscle
Myoglobin & heart-type fatty acid binding protein are smaller molecules that may appear in circulation as early as 30 min after MI, but lack of specificity limits utility
Does it reflect true myocardial injury? Almost always the case for Tn; CK-MB less specific.
If myocardial injury, what is the pathobiology? Ddx includes:
>99th %ile w/ ≥1 of the following:
1) sx of ischemia
2) new Qw
4) intracoronary thrombus
5) imaging evidence of new loss of myocardium or regional wall motion abnl non-ischemic injury (eg, myocarditis/toxic CMP, cardiac contusion) multifactorial (eg, PE, sepsis, severe HF, renal failure, Takotsubo, infiltrative disease)
If MI, what type? (Circ 2012;126:2020)
Type
Descriptor
Features
1
Spontaneous
Pathologic process in wall of coronary artery (eg, plaque rupture) resulting in intraluminal thrombus
2
Supply-demand mismatch not due to Δ in CAD
Mismatch due to, eg, ↑ ↑ or ↓↓ HR or BP, profound anemia or hypoxemia, coronary vasospasm, HCM, severe AS
3
Sudden cardiac death
4a
Related to PCI
Tn >5x 99th %ile or >20% rise if already elevated +≥1 MI clinical/imaging criteria above
4b
Stent thrombosis
5
Related to CABG
Tn > 10x 99th %ile + new Qw or LBBB, angio confirmation, or new wall motion abnl
Treadmill electrocardiography: can be performed after 6-8 h of evaluation
Radionuclide imaging in Pts who cannot exercise or have uninterpretable ECG
Can perform acute rest perfusion imaging if ongoing or recent (w/in 2 h) pain ([circled dash] scan r/o ischemia; ⊕ scan could represent ischemia or infarct, need pain-free rest images)
Echo (w/ or w/o stress) to assess for regional wall motion abnl; interpretation difficult in those w/ h/o prior MI
Coronary CT angio (CCTA): NPV 98% for signif CAD, but PPV 35% for ACS; helpful to r/o CAD if low-intermed prob of ACS. CCTA vs noninvasive fxnal test for ischemia → ↓ time to dx & LOS, but ↑ probability of cath/PCI, contrast exposure & ↑ radiation (NEJM 2012;366:1393 & 367:299; JACC 2013;61:880)
Indications: dx CAD, evaluate Δ in clinical status in Pt w/ known CAD, risk stratify s/p ACS, evaluate exercise tolerance, localize ischemia (imaging required)
Contraindications (Circ 2002;106:1883; & 2012;126:2465)
Absolute: AMI w/in 48 h, high-risk UA, acute PE, severe sx AS, uncontrolled HF, uncontrolled arrhythmias, myopericarditis, acute aortic dissection
Typically via treadmill
Protocol
Description
Standard Bruce
↑ speed & incline q3min until 85% max predicted HR or sxs
Modified Bruce
Adds two stages at start that require less work than standard Bruce stage 1; consider in sedentary/deconditioned Pt
Submaximal
Stop earlier (eg, 70% max predicted HR or 5 METs or any anginal sx); consider if recent MI
Stationary cycle or arm ergometry (lower max workload) if Pt cannot walk
Preferred if LBBB or V-paced, as higher prob of false ⊕ exercise imaging (typically reversible or fixed anteroseptal defect).
Coronary vasodilator: diffuse coronary arteriolar vasodilation → relative “coronary steal” from vessels w/ fixed epicardial disease. Reveals CAD, but not if Pt ischemic w/ exercise. Options include:
Adenosine or dipyridamole (↑ adenosine reuptake). Side effects: flushing, bradycardia & AVB, dyspnea & bronchospasm.
Selective A2A receptor agonist (eg, regadenoson): less flushing, dyspnea, bronchospasm
Longer half-life of dipyridamole & regadenoson allow to be combined w/ exercise
Avoid caffeine (adenosine receptor antagonist) w/in 12 h of test. Conversely, can use aminophylline to reverse effects of these agents.
Chronotropes/inotropes (more physiologic): dobutamine (may precip tachyarrhythmia)
Use if uninterpretable ECG (V-paced, LBBB, resting ST ↑ >1 mm, digoxin, LVH, WPW), after indeterminate ECG test, or if pharmacologic test
Use when need to localize ischemia (often used if prior coronary revasc)
Ideally use exercise as stress modality rather than pharmacologic
ECG-gated imaging allows assessment of LV fxn (including regional systolic wall thickening or lack thereof as a sign of ischemia/infarction)
HR (must achieve ≥85% of max pred HR [220 – age] for exer. test to be dx), BP response, peak double product (HR × BP; nl >20k), HR recovery (HRpeak – HR1 min later; nl > 12)
Max exercise capacity achieved (METS or min)
Occurrence of symptoms (at what level of exertion and similarity to presenting sx)
ECG Δs: downsloping or horizontal ST ↓ (≥1 mm) 80 ms after QRS predictive of CAD upsloping ST ↓ w/ rapid return to baseline usually due to ↑ HR & atrial repol artifact lead V5 most sensitive; Δs isolated to inferior leads may be artifact location of ST ↓ does not localize ischemic territory
STE: highly predictive of CAD & localizes; in aVR suggests LM CAD; nonspecific if in leads w/ prior Qw
Duke treadmill score = exercise min – (5 × max ST dev) – (4 × angina index) [angina index = 0 none, 1 nonlimiting sxs, 2 limiting sxs]
Category
1-y mort
5-y survival
Low risk (≥5)
60% w/o signif stenosis
<1%
97%
Moderate risk (-10 to 4)
2-3%
90%
High risk (≤-11)
≥5%
65%
Imaging: radionuclide defects or echocardiographic regional wall motion abnormalities reversible defect = ischemia; fixed defect = infarct; transient isch dilation = severe CAD false ⊕: breast → ant “defect” and diaphragm → inf “defect” false [circled dash] may be seen if balanced (eg, 3VD) ischemia (global ↓ perfusion w/o regional Δs)
Viable myocardium = dysfxn at rest, but not scarred and has potential for recovery
Goal: identify hibernating or stunned myocardium that could regain fxn after revasc
Viability Test
Sens
Spec
Mechanism
˜85%
˜75%
Assesses cellular integrity
˜90%
˜65%
Assesses cell metabolism
Dobut stress echo
˜80%
˜80%
Assesses contractile reserve
Myocard contrast echo
˜85%
˜50%
Assesses cellular integrity
Thallium: rest-redistrib or stress-reinjection
˜85%
˜55%
Assesses cellular integrity (K+ analogue taken up by Na/K-ATPase)
Technetium-MIBI
˜85%
˜65%
Assesses cellular integrity (requires intact mitochondria)
In Pts presenting with CP, presence of plaque sensitive (100%) but not specific (54%) for ACS, NPV 100%, PPV 17% (JACC 2009;53:1642). CCTA vs noninvasive fxnal test for ischemia → ↓ time to dx & LOS, but ↑ probability of cath/PCI, contrast exposure & ↑ radiation (NEJM 2012;366:1393 & 367:299; JACC 2013;61:880).
NPV 100%, PPV 17% (JACC 2009;53:1642). CCTA vs noninvasive fxnal test for ischemia → ↓ time to dx & LOS, but ↑ probability of cath/PCI, contrast exposure & ↑ radiation (NEJM 2012;366:1393 & 367:299; JACC 2013;61:880).
In sx outPt, CCTA vs fxnal testing led to more radiation, coronary angiography & revascularization, but no difference in clinical outcomes (PROMISE NEJM 2015;372:1291)
CCTA images may be limited if: HR >60-70 bpm, irregular rhythm, calcium deposition or stents (may create artifact), inability to breath hold 5 sec, vessel diameter < 1.5 mm. Image quality best at slower & regular HR (? give βB if possible, goal HR 55-60).
Useful for assessing patency of bypass grafts
Unlike CCTA, does not require iodinated contrast, HR control or radiation exposure. Can also assess LV fxn, enhancement (early = microvasc obstruction; late = MI). Limitations: cost, operator-dependent, long duration, ↓ spatial resolution.
In head-to-head comparisons, CMRI and CCTA appear to have grossly comparable sensitivity and specificity (JACC 2005;46:92; Annals 2006;145:207)
Quantifies extent of calcium; thus estimates plaque burden (but not % coronary stenosis)
CAC Score
Suggested plaque burden
0
No disease
1-99
Mild disease
100-399
Moderate disease
>400
Severe disease
? value as screening test to r/o CAD in sx Pt (CACS <100 → 3% probability of signif CAD; but interpretation affected by age, gender)
Pretest Likelihood of CAD (%) (NEJM 1979;300:1350; Annals 1993;118:81)
Nonanginal (≤1 sx)
Atypical angina (2 sx)
Typical angina (all 3 sx)
Age
Men
Women
Men
Women
Men
Women
35
3↔ 35
1 ↔ 19
8 ↔ 59
2 ↔ 39
30 ↔ 88
10 ↔ 78
45
9 ↔ 47
2 ↔ 22
21 ↔ 70
5 ↔ 43
51 ↔ 92
20 ↔ 79
55
23 ↔ 59
4 ↔ 21
45 ↔ 79
10 ↔ 47
80 ↔ 95
38 ↔ 82
65
49 ↔ 69
9 ↔ 29
71 ↔ 86
20 ↔ 51
93 ↔ 97
56 ↔ 84
Classic angina sx: substernal chest pain, provoked by exertion, relieved by rest or NTG. W/in each cell, 1st # is % for Pt w/o risk factors; 2nd is for Pt w/ DM, smoking, & hyperchol.
Noninvasive testing (qv) if intermediate risk (ie, >10-20% & <80-90%). If Pt has low prob, then ↑ risk false ⊕; if very high prob, [circled dash] test does not adeq r/o CAD, consider cor angio.
consider cor angio.
Coronary angio if: high-risk noninv results (qv); very high pretest prob; refract angina; uncertain dx after noninvasive testing (& compelling need to determine dx), occupational need for definitive dx (eg, pilot) or inability to undergo noninvasive testing; survivor of SCD or life-threatening vent. arrhythmia; unexplained heart failure or ↓ EF; suspected spasm or nonatherosclerotic cause of ischemia (eg, anomalous coronary)