, Domenico Corrado2 and Cristina Basso1
(1)
Cardiovascular Pathology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
(2)
Cardiology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
Sudden cardiac death (SCD) is mostly ascribable to coronary artery disease and is due to a large spectrum of both congenital and acquired morbid entities [1–3]. Premature coronary atherosclerosis is ranking first even in young age, with peculiar features of an accelerated proliferative phenomenon [2, 4].
3.1 Atherosclerotic Coronary Artery Disease
The disease and the mechanisms of cardiac arrest in the young are peculiar insofar as:
1.
2.
The phenomenon is mostly focal, consisting of a single vessel disease in contrast with the adult and elderly population where a diffuse, multivessel involvement is typically found (Fig. 3.1). The obstructive (>75 % lumen stenosis), usually eccentric, atherosclerotic plaque is often located in the proximal tract of the anterior descending branch of the left coronary artery [2, 4] (Fig. 3.2).
3.
The culprit lesion rarely shows the classic features of vulnerable atherosclerotic plaque (necrotic core covered by a thin fibrous cap) [1, 5–8] (Figs. 3.3 and 3.4). It is mostly fibrocellular, due to recent smooth muscle cell growth indicating an accelerated proliferative phenomenon [2, 4, 9–15] (Figs. 3.1, 3.5, 3.6, and 3.7).
4.
When thrombotic occlusion is observed, the thrombosis rarely precipitates as a consequence of plaque rupture or fissuring of the atherosclerotic plaque with thin fibrous cap [2, 4] (Figs. 3.8, 3.9, and 3.10). The thrombus is a mixture of platelets and fibrin entrapping red cells (Fig. 3.11). More frequently, endothelial erosion accounts for thrombogenicity [16, 17] (Figs. 3.12 and 3.13) and inflammation of the superficial layer of the intima (“endothelitis”) is often observed as a cause of endothelial cell detachment (Fig. 3.14). In the setting of endothelial erosion due to extensive T-lymphocytes and macrophage infiltration, molecular pathology investigation can help in detecting viral infection as a cause of coronary plaque instability precipitating thrombotic occlusion [18] (Fig. 3.15). The thrombosis may be occlusive or mural (Fig. 3.16). In both conditions, detachment of thrombus fragments may lead to embolism into the distal coronary small arteries (Fig. 3.17).
5.
In the absence of an organic occlusion of a coronary artery, as in the case of a single obstructive atherosclerotic plaque, a transient ischemic attack, most probably due to vasospasm, has been demonstrated to occur just before cardiac arrest, with ST segment elevation on ECG like in Prinzmetal variant angina [12, 14, 19, 20] (Figs. 3.18 and 3.19). Reperfusion, following release of vasospasm, entails life-threatening electrical instability, because of massive calcium cellular inflow, due to damage of sarcolemma following transient myocardial ischemia.
6.
Risk factors for accelerated atherosclerotic coronary artery disease leading to SCD in the young are not easily recognizable, due to the almost absent clinical information, including laboratory tests, in this apparently healthy population. Among the risk factors, cocaine and other drug addiction are well known particularly in young adults [21, 22]. Systematic toxicology investigation indicated that about 3 % of SCD are cocaine-related, and premature coronary artery atherosclerosis, with or without lumen thrombosis, is a frequent finding that may account for myocardial ischemia at risk of cardiac arrest in cocaine addicts [21] (Fig. 3.20). Small vessel disease is quite often associated with epicardial atherosclerotic coronary artery disease in this population (Fig. 3.21).
7.
In our extensive experience of SCD in the young, an overt acute myocardial infarction was rarely observed, even at histology, and myocardial scar due to previous infarction was also quite rare (Fig. 3.22) [4], in contrast with the adult elderly population [1, 23, 24] (Figs. 3.1 and 3.23). Because of the instantaneous death, it is impossible to predict whether these young people would have developed overt myocardial infarction, if resuscitated. Certainly, in case of fresh occlusive thrombosis, the occurrence of cardiac arrest due to ventricular fibrillation is most probably equivalent to that occurring within the first hour of myocardial infarction with out-of-the-hospital fatal outcome [25–28]. In case of transient coronary artery occlusion following vasospasm, it is also impossible to establish whether the constriction time would have been long enough to precipitate an acute myocardial infarction, either subendocardial or even transmural. It is well known that histological evidence of myocardial infarction is detectable not before 3–4 h from the time of coronary occlusion. It is highly probable that, like in experimental ligation of the descending coronary artery in the dog which immediately triggers ventricular fibrillation, transient ischemia due to vasospasm may be enough to threaten the regular electrical impulse transmission or favor ectopic ventricular tachycardia by triggered activity. A genetic predisposition to ventricular fibrillation has been suggested with a specific single nucleotide polymorphism in chromosome 21q21 [29]. A pathological substrate in the myocardium, consistent with a reentry mechanism for ventricular arrhythmias, is lacking in the absence of fibrous scars (Fig. 3.23) and the hypothesis that transient ischemia may precipitate an ion channel disorder cannot be ruled out.
Summing up, atherosclerotic SCD in the young is mostly due to a vasospastic transient coronary occlusion at the level of a single non-atheromatous obstructive plaque, located in the first tract of the left anterior descending branch (i.e., functional plaque instability in the form of vasospasm). When coronary artery occlusion is due to thrombosis, the latter occurs more frequently upon endothelial erosion rather than fibrous cap rupture. Gross and/or histological evidences of acute/chronic myocardial infarction are rare.
3.2 Non-atherosclerotic Coronary Artery Disease
One third of the cases of fatal coronary artery disease in the young are non-atherosclerotic and can be either acquired or congenital, with coronary artery anomalies being the most frequent form [30–32].
3.2.1 Embolism
Embolism may complicate cardiac disease of the left-sided heart with mural thrombosis (atrial fibrillation, dilated cardiomyopathy, acute and chronic myocardial infarction) and the coronary arterial tree may be the target [33] (Fig. 3.24). However, these conditions are extremely rare in the young and athletes. More frequently, a coronary embolism may occur in the presence of left atrial myxoma (Fig. 3.25) or endocardial fibroelastoma [34–36]. While in the setting of cardiac myxoma the embolism may be neoplastic because of the detachment of myxoid tissue from a villous and friable mass, in the setting of endocardial fibroelastoma the embolus is mostly thrombotic in nature because of stratification of fibrin over or within the fronds of the tumor, followed by detachment. In contrast with the villi of myxoma, the fronds of a papilloma present with a firm fibroelastic stalk, almost impossible to detach. However, tumor embolization cannot be excluded, since rare cases have been reported with distal embolism and histological evidence of neoplastic nature even in cases with endocardial fibroelastic papilloma. When the tumour grows upon a coronary aortic cusp, it may herniate into and occlude the coronary ostium, thus precipitating cardiac arrest [37]. Septic coronary embolism may occur in the setting of infective endocarditis and cause SCD, but of course this does not usually occur unexpectedly in a healthy subject [38] (Fig. 3.26).
3.2.2 Arteritis
Necrotizing noninfectious arteritis may involve the coronary arteries and account for coronary occlusion and SCD. This is the case of Kawasaki disease, which is featured by a peculiar involvement of the coronary arterial tree and characterized by a tendency to aneurysmal formation, because of tunica media inflammatory necrosis, and lumen thrombotic occlusion, leading to myocardial infarction and chronic ischemic heart disease [39–41] (Fig. 3.27).
Giant cell arteritis may also involve the coronary arteries [42, 43]. With specific reference in the young, this is the case of Takayasu’s arteritis which usually affects young women and involves the aortic arch and brachiocephalic arteries with severe stenosis and subclavian “steal.” The intrapericardial great arteries may be involved as well. When the ascending aorta is affected, the inflammation may be extended to the coronary ostia with lumen obstruction, so severe to precipitate myocardial ischemia and SCD (Fig. 3.28). In contrast of Kawasaki disease, it is not associated with aneurysm because of peri-adventitial fibrotic coating.
3.2.3 Dissection
It is a coronary disease typical of young–middle-aged women, frequently occurring in the peripartum period [44–47]. It is characterized by sudden lumen occlusion because of dissecting hematoma of the tunica media of the main subepicardial coronary arteries (Fig. 3.29), usually the left trunk and the descending coronary artery, but also the right coronary artery and other coronary branches. The hematoma pushes the intimal-inner-medial layers into and occludes the true lumen [44, 47] (Figs. 3.30 and 3.31). Sudden cardiac arrest is the frequent clinical presentation, as it is in coronary occlusion by thrombosis upon atherosclerotic plaque or by coronary embolism.
In contrast with the aorta, where dissection represents the most frequent form of acute aortic syndrome, SCD in coronary dissection is not “mechanical” due to external rupture with cardiac tamponade. On the opposite, it occurs through an acute myocardial ischemia with ventricular fibrillation. The pathogenesis of dissection is controversial. The “fragility” of the coronary tunica media is not comparable to that of the aorta. The coronary artery wall is muscular, the aortic one is elastic. Erdheim’s cystic medial necrosis is rarely observed in coronary dissection. The only peculiar, frequently reported, histological feature is an eosinophilic inflammatory infiltrate in the outer tunica media and adventitia as to advance the hypothesis of an allergic arteritis [44, 48, 49] (Fig. 3.31). Its significance remains obscure since coronary dissection is not a typical finding in allergic syndromes and the eosinophilic infiltrate is mostly confined to the dissected coronary segment.
Also the source of the blood dissecting the coronary wall is controversial. In contrast with the thoracic aorta, there are no vasa vasorum in the coronary arterial wall to potentially account for intramural hematoma. The finding of an intimal tear is quite rare at postmortem in spontaneous coronary dissection, probably because technical difficulties to detect [44] (Fig. 3.32). On the contrary, it is regularly seen in iatrogenic cases complicating catheter maneuvers, like selective coronary angiography, angioplasty, and cannulation of coronary ostia for cardioplegia during surgery. Nonetheless, angiography of coronary dissection typically shows a “binary” appearance (true and false lumen), which clearly suggests an intimal entry into the false lumen. Exceptionally, cystic medial necrosis, similar to that observed in aortic dissection, can be detected (Fig. 3.33). Finally, external factors like trauma or drug abuse should be carefully investigated. In particular, cocaine addiction has been even associated with coronary dissection. The elevated wall stress, due to increased arterial blood pressure from cocaine’s inotropic and chronotropic effects combined with its direct vasoconstrictive effect, may be responsible for the formation of an intimal tear and the subsequent dissection of the coronary artery [50].
3.3 Congenital Coronary Artery Anomalies
Anomalies of the origin and course of the major coronary arteries may account of myocardial ischemia and SCD especially during effort [51–56].
In the normal heart, the coronary arteries arise from the left and right anterior sinuses of Valsalva of the aorta, perpendicular to the aortic wall. The pulmonary root, which is anterior and to the left of the aorta, does not interfere with the origin and proximal course of the left and right coronary arteries [57] (Figs. 3.34 and 3.35).
Major anomalies, like the origin of the left or, more rarely, the right coronary artery from the pulmonary trunk (Figs. 3.36 and 3.37), have been reported in cases of juvenile or infant SCD [55]. The origin of a coronary artery from the pulmonary trunk accounts of an aortic–pulmonary fistula: the different resistance between the systemic and pulmonary blood circulation entails a left-to-right shunt with a blood steal from the myocardium, severe myocardial ischemia, and necrosis [58]. The shunt and steal is not present during fetal life, when the systemic and pulmonary resistances are equal. The situation precipitates at birth, at the time of closure of the ductus arteriosus, with the onset of lung respiratory function and fall of pulmonary vascular resistance. Then, the anomalous origin of a coronary artery from the pulmonary trunk may be so severe as to become incompatible with the postnatal blood circulation, because of the ischemic myocardial injury. Cases, however, have been reported with long-term survival as to benefit surgical repair with reconnection of the anomalous coronary artery to the aorta [59, 60].
Other apparently minor, and more frequent, coronary artery anomalies are life-threatening in adolescence and young age. They may be classified into four major categories, with different degrees of certainty (i.e., certain, highly probable, or uncertain) in defining the cause–effect relationship between the anomaly and the SCD event [61]:
1.
Origin of a main coronary artery from the opposite (wrong) sinus. This is the case of origin of the right coronary artery from the left aortic sinus or origin of the left coronary artery for the right anterior sinus [62–65] (Figs. 3.38 and 3.39). The latter is much more malignant because the anteroseptal and lateral walls of the left ventricle are perfused by this artery, whereas the former has been reported also as incidental finding in noncardiac deaths. They may be well visualized in vivo by coronary angiography or even noninvasively by two-dimensional echocardiography, cardiac magnetic resonance or computed axial tomography [65]. The anomalous origin of the coronary artery from the wrong sinus is a cause of ischemia, especially during effort, through several mechanisms (Figs. 3.40, 3.41, and 3.42):
(a)
An obtuse angle takeoff from the aorta
(b)
A slit-like lumen
(c)
The anomalous vessel running between the aorta and the pulmonary artery
(d)
The intramural aortic course of the proximal anomalous coronary artery
They all contribute to precipitate a discrepancy between coronary blood flow demand during effort and effective coronary blood perfusion across the stenotic proximal coronary segment. Repeated prolonged efforts create focal myocardial ischemic injury that with time precipitates patchy, highly arrhythmogenic fibrotic scars (Fig. 3.43). Combination of acute and chronic myocardial damage is a cocktail of malignant substrate with life-threatening electrical turmoil.
2.
Origin of the left circumflex artery from the right coronary artery or directly from the right sinus of Valsalva (Fig. 3.44). This is the most frequent coronary anomaly and a source of controversy, because it is usually considered a benign variant. However, cases in which this coronary anatomy pattern was the only abnormality found at autopsy of SCD (Fig. 3.45) have been observed, associated even with myocardial infarction just in the related left lateral myocardial region and in the absence of atherosclerotic obstructive disease [66, 67] (Fig. 3.46).
3.
High takeoff of a coronary artery. Usually the coronary ostia are located just underneath or above the sino-tubular junction [68] (Fig. 3.35). There are cases of SCD, in which the sole abnormality is an origin of the coronary artery from the tubular part of the aorta, 1–2 cm above the sino-tubular junction [53, 54] (Figs. 3.47 and 3.48). This is usually the case of the right coronary artery, which, by reaching the right AV groove, presents with a vertical course within the aortic tunica media, a condition which clearly impairs coronary blood flow at the time of increased demand during effort, as it is with a coronary artery arising from a wrong sinus.
4.
Myocardial bridge. Intramyocardial course of a major coronary artery, particularly the left anterior descending branch, is quite common (nearly 30 % of people) as to be considered a variant of normal. There are cases, however, with angina and ECG ST segment alterations, in the absence of other explanation but a “milking effect” at coronary angiography. The intramyocardial course is deep and long, constricted also during diastole, when coronary perfusion occurs [69]. Histologically, the coronary segment is not simply covered by a myocardial fascicle (“bridge”) but also surrounded by a sheath of myocardium acting as sphincter [70] (Figs. 3.49 and 3.50).
In the AECVP guidelines for the investigation of SCD, this substrate is classified among those of uncertain significance in terms of risk [61]. Noteworthy, by reviewing with attention the illustrations of reported cases of SCD with myocardial bridge, they were cases of hypertrophic cardiomyopathy with myocardial bridge [71, 72] and now it is well known that myocardial bridge is part of the phenotypic expression of hypertrophic cardiomyopathy [73].
3.4 Image Gallery
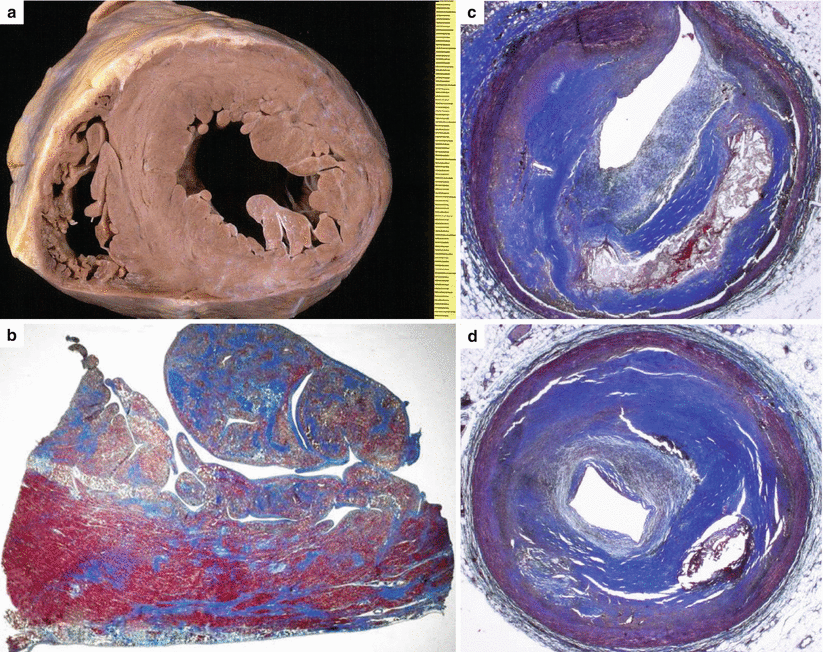
Fig. 3.1
Arrhythmic sudden cardiac death in the adult. A 58-year-old man with previous myocardial infarction due to multivessel atherosclerotic coronary artery disease. (a) Transverse section of the heart specimen showing thinning of the posterolateral left ventricular free wall with scarring. (b) Subendocardial and patchy transmural replacement-type fibrosis is visible on panoramic histological section of the posterolateral left ventricular free wall (Heidenhain trichrome). (c, d) Transverse sections of the anterior descending and circumflex branches of the left coronary artery show critical stenosis due to fibro-atheromatous plaques (Heidenhain trichrome)
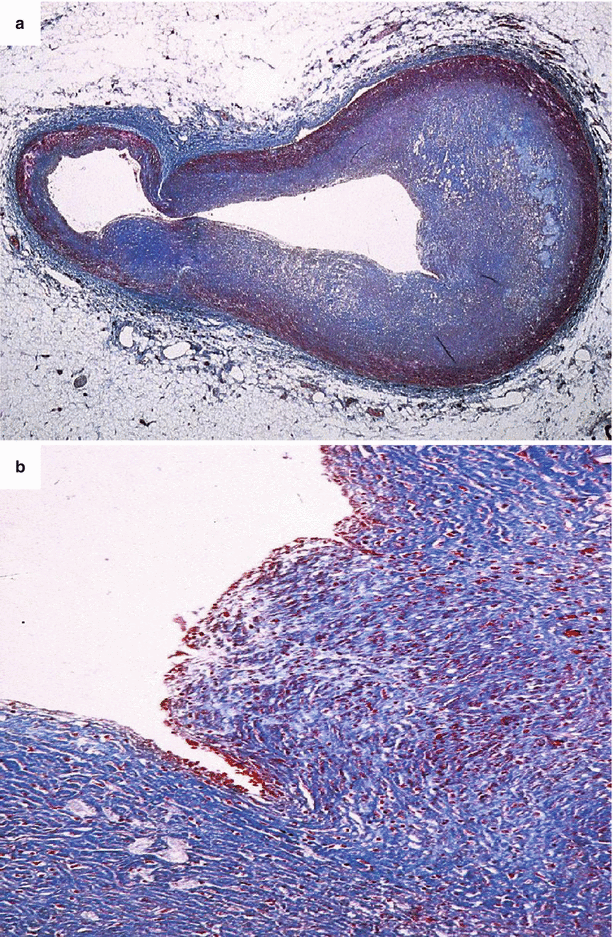
Fig. 3.2
Arrhythmic sudden cardiac death in a 30-year-old man due to atherosclerotic disease of the proximal left anterior descending coronary artery. (a) Histology showing an obstructive eccentric fibrous plaque at the origin of the first diagonal branch. Note the preserved tunica media (Heidenhain trichrome). (b) Close-up of a, showing a layer of intimal smooth muscle cells hyperplasia (Heidenhain trichrome)
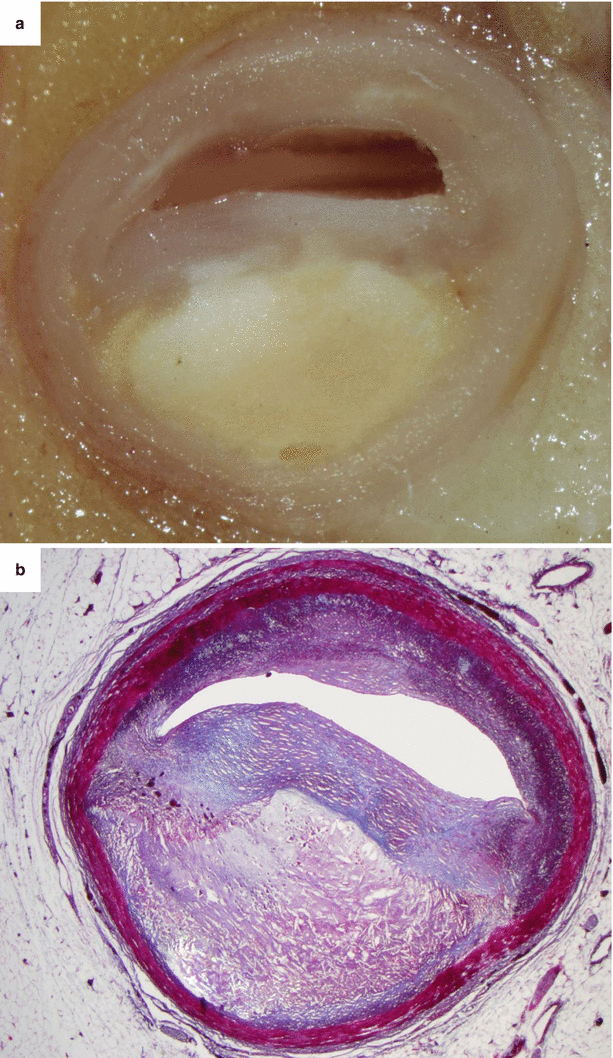
Fig. 3.3
Arrhythmic sudden cardiac death due to vulnerable obstructive atherosclerotic plaque of the proximal left anterior descending coronary artery in the adult. A 56-year-old man. (a) Macroscopic examination of the proximal left anterior descending coronary artery showing an eccentric plaque with a big yellow core and a gray fibrous cap. (b) Histology of a vulnerable fibro-atheromatous plaque (Heidenhain trichrome)
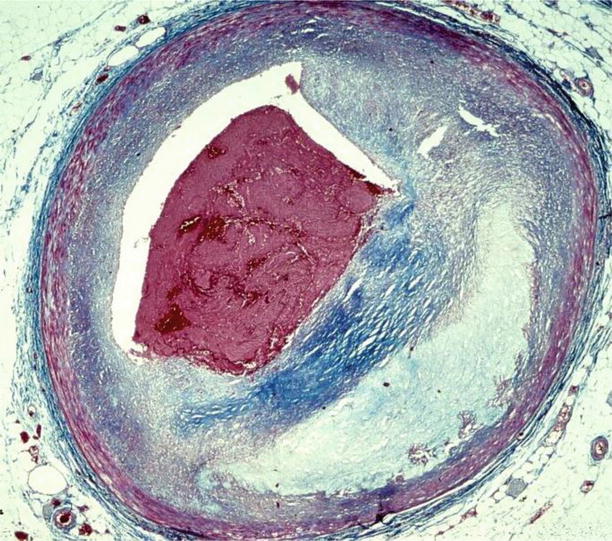
Fig. 3.4
Arrhythmic sudden cardiac death due to fresh occlusive thrombosis of the proximal left anterior descending coronary artery, superimposed upon an eccentric fibro-atheromatous plaque with a large lipid core and superficial erosion in a 33-year-old man (Heidenhain trichrome)
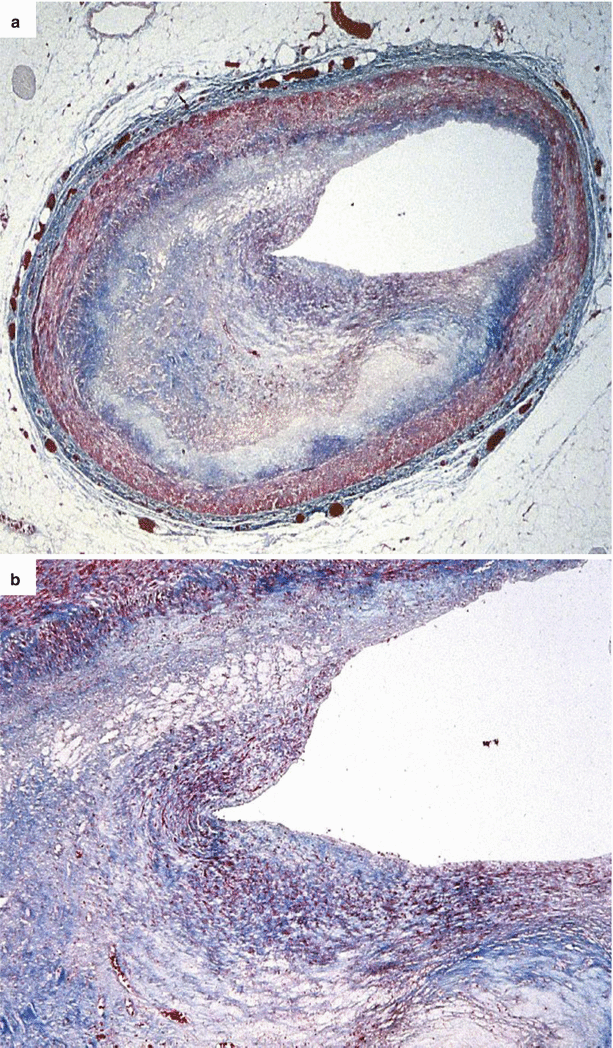
Fig. 3.5
Arrhythmic sudden cardiac death due to single vessel coronary artery disease in a 35-year-old man. (a) An eccentric fibrous fibro-atheromatous plaque, rich in foam cells, is located in the proximal left anterior descending coronary artery (Heidenhain trichrome). (b) Close-up: a layer of intimal smooth muscle cells hyperplasia is visible on the luminal side (Heidenhain trichrome)
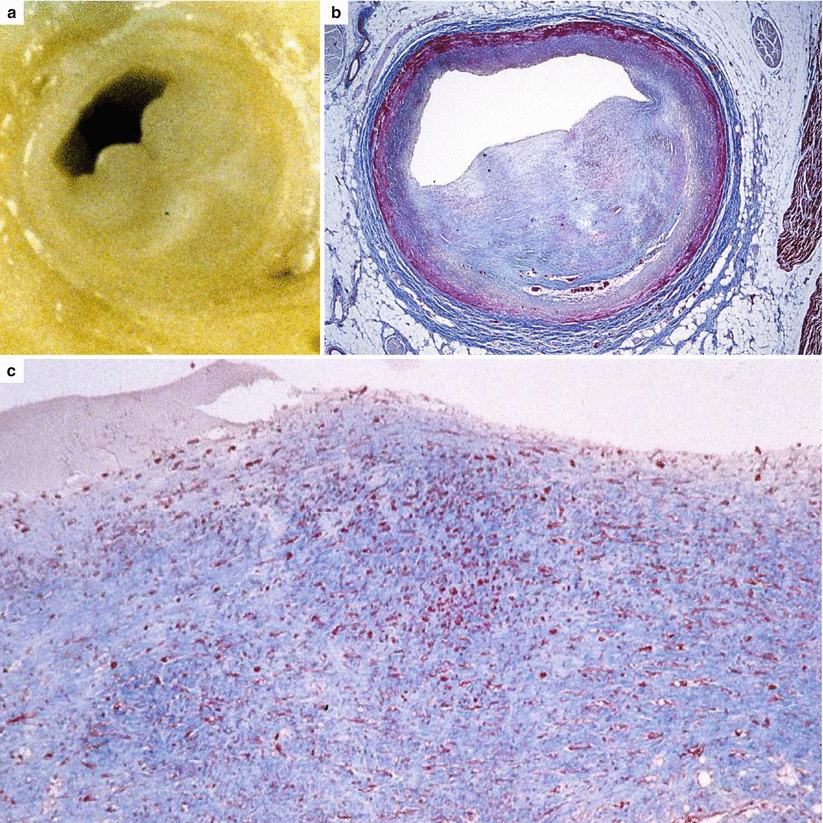
Fig. 3.6
Arrhythmic sudden cardiac death due to single vessel coronary artery disease in a 25-year-old man. (a) Macroscopic examination of the proximal left anterior descending coronary artery shows an eccentric, gray plaque. (b) At histology, the fibrocellular nature of the plaque, in the absence of a lipid core, is visible (Heidenhain trichrome). (c) At higher magnification, note a layer of intimal smooth muscle cells hyperplasia on the luminal side (Heidenhain trichrome)
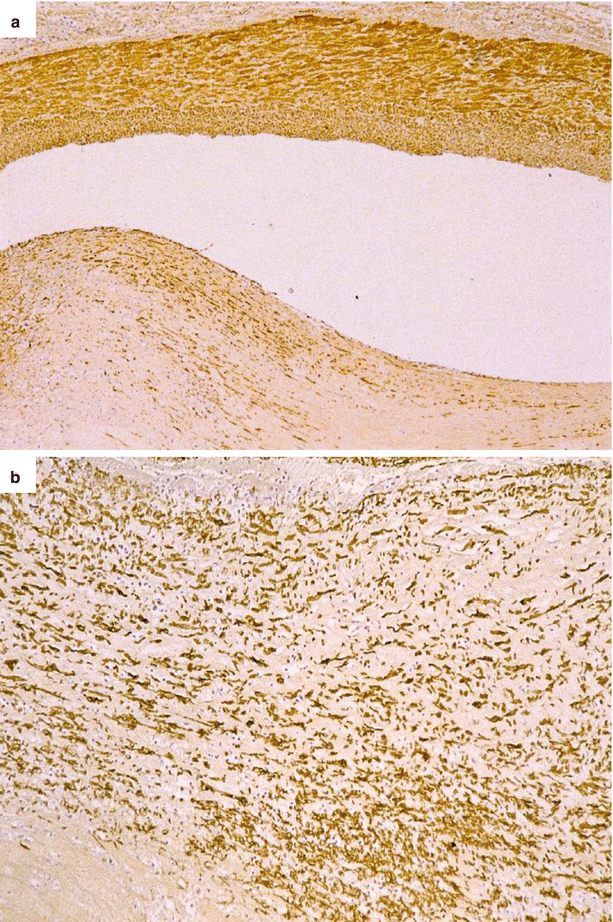
Fig. 3.7
Immunohistochemistry of an obstructive eccentric fibrocellular plaque located at the level of the proximal left anterior descending coronary artery in a 32-year-old man who died suddenly. (a) The intimal cell hyperplasia on the cap of the plaque (bottom) consists of smooth muscle cells, since they show an immunoreactivity for smooth muscle actin (SMA) antibody similar to the cells of the media (top) (SMA antibody). (b) Close-up of the intimal cell hyperplasia, at the top of the plaque (SMA antibody)
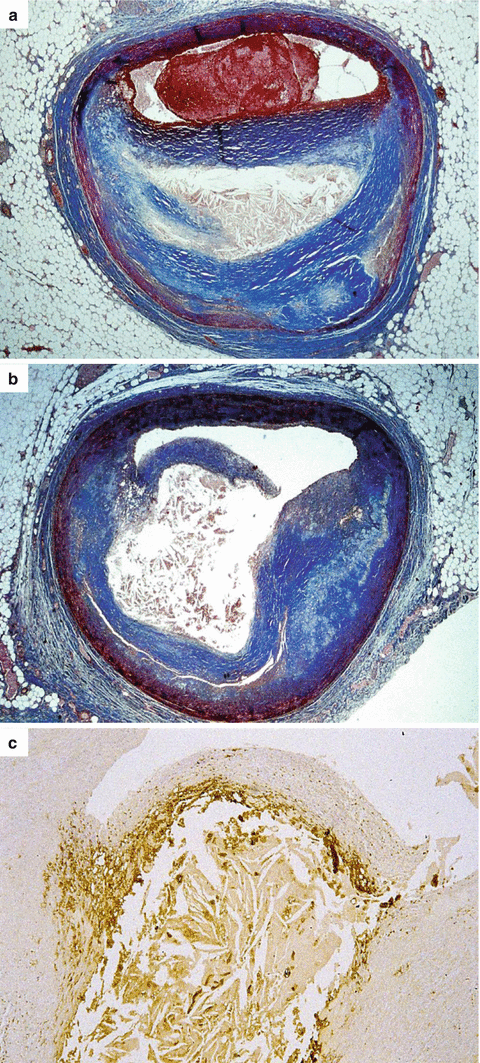
Fig. 3.8
Arrhythmic sudden cardiac death caused by occlusive thrombosis of the left anterior descending coronary artery in a 34-year-old man due to plaque rupture. (a) The acute luminal thrombosis is visible upon a fibro-atheromatous plaque (Heidenhain trichrome). (b) On the adjacent, parallel, coronary artery section, a rupture at the level of the shoulder region of the thin fibrous cap is visible (Heidenhain trichrome). (c) By immunohistochemistry, the fibrous cap appears massively infiltrated by macrophages (CD68 antibody)
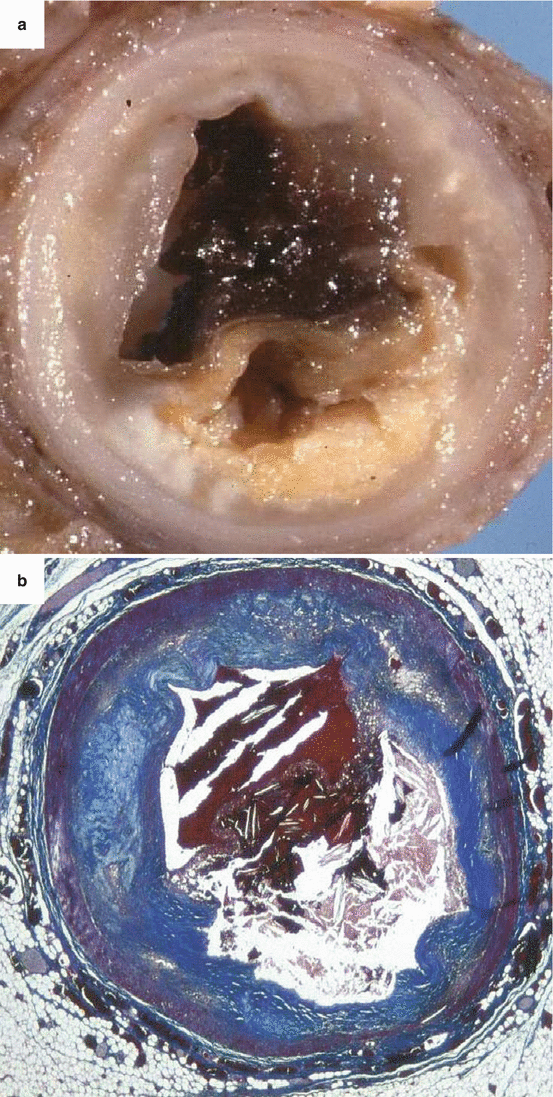
Fig. 3.9
Arrhythmic sudden cardiac death caused by occlusive thrombosis of the left anterior descending coronary artery in the adult, a 55-year-old man with plaque rupture. (a) Gross view of the culprit coronary segment showing the acute luminal thrombosis upon a nonobstructive atherosclerotic plaque. (b) On the corresponding histological slide of the fibroatheromatous plaque, abundant cholesterol clefts in the necrotic core and the rupture of a thin fibrous cap with occlusive thrombosis are visible (Heidenhain trichrome)
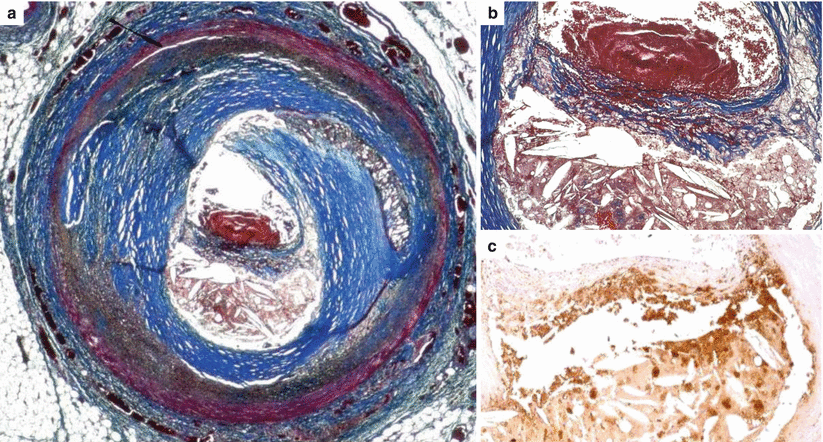
Fig. 3.10
Arrhythmic sudden cardiac death caused by occlusive thrombosis of the left anterior descending coronary artery in the adult, a 60-year-old man with plaque rupture. (a) Histological section of the culprit coronary segment showings the acute luminal thrombosis complicating an obstructive fibro-atheromatous plaque with a thin, disrupted, fibrous cap (Heidenhain trichrome). (b) Close-up of a, shows abundant cholesterol clefts and hemorrhage in the necrotic core and the thin, disrupted, fibrous cap (Heidenhain trichrome). (c) Same field as in (b), note the massive infiltration by macrophages of the thin fibrous cap as well as of the necrotic core (CD68 antibody), releasing matrix metalloproteinases
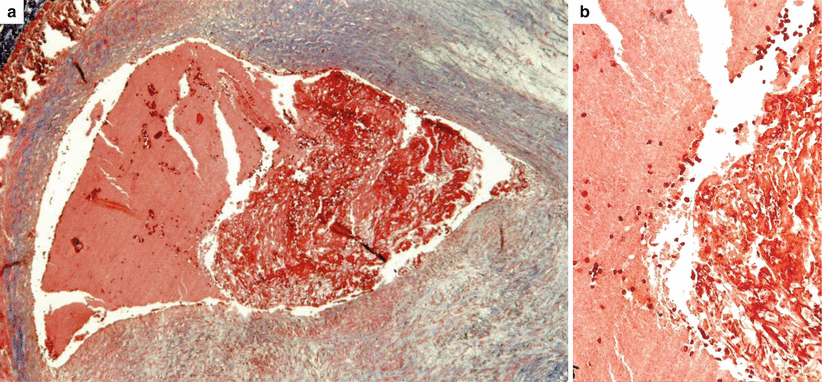
Fig. 3.11
Arrhythmic sudden cardiac death due to occlusive thrombosis of the left anterior descending coronary artery (culprit lesion) in a 40-year-old woman. (a) The thrombus is a mixture of platelets (left) and fibrin (right) entrapping red cells (Heidenhain trichrome). (b) Close-up of a, showing the border between platelets and fibrin network (Heidenhain trichrome)
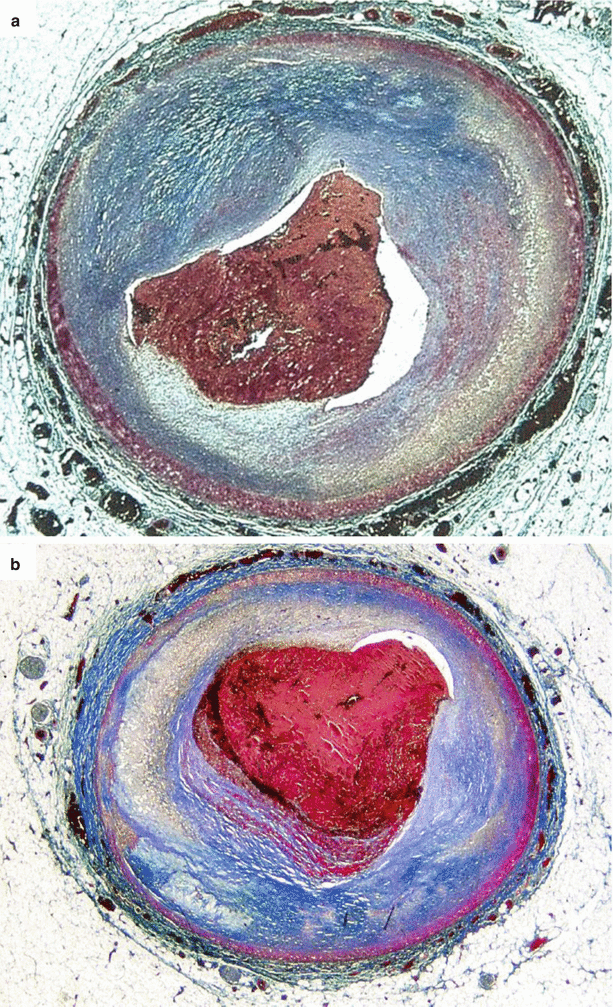
Fig. 3.12
Arrhythmic sudden cardiac death due to occlusive thrombosis of the left anterior descending coronary artery with plaque erosion in two 30-year-old men. (a, b) Fibrocellular, nonobstructive, eccentric atherosclerotic plaques, devoid of lipids, are complicated by occlusive thrombosis, in the absence of fibrous cap rupture (so-called plaque “erosion”) (Heidenhain trichrome)
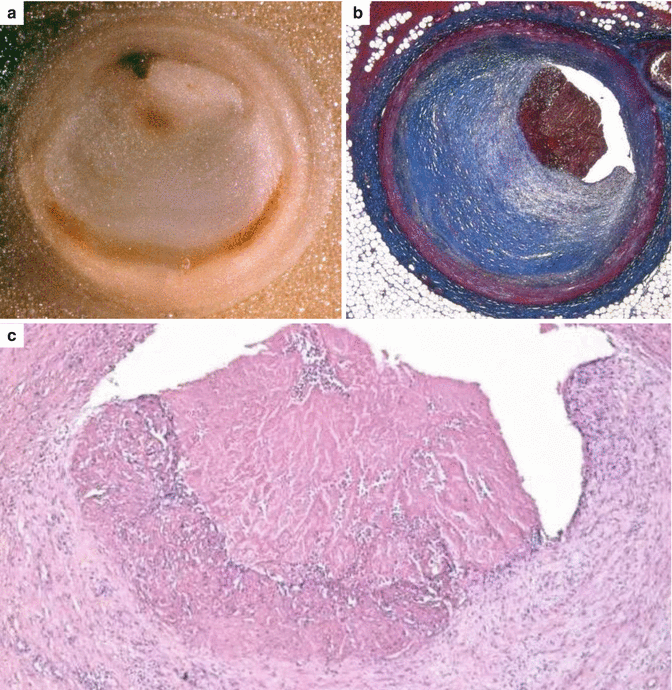
Fig. 3.13
Arrhythmic sudden cardiac death due to occlusive thrombosis of the left anterior descending coronary artery with plaque erosion in a 31-year-old man, with a history of drug abuse (cannabis and cocaine). (a) Macroscopic section of the culprit coronary artery showing an occlusive thrombosis upon an eccentric, gray plaque with critical stenosis. (b) Corresponding histology with evidence of a fibrocellular atherosclerotic plaque, devoid of lipids, complicated by occlusive thrombosis (Heidenhain trichrome). (c) At higher magnification, two separate layers of thrombus deposition are visible, i.e., fresh and subacute with early organization (hematoxylin–eosin)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


