Coronary Artery Bypass Grafts
Allen P. Burke, M.D.
Fabio R. Tavora, M.D., Ph.D.
Clinical
Incidence and Indications
Coronary artery bypass graft surgery is performed 350,000 times annually in the United States.1 The general indications are multivessel or left main coronary disease.2,3 Recently, there has been a shift toward percutaneous revascularization for multivessel disease as well as single-vessel stenoses, suggesting that the rate of coronary artery bypass graft surgery may decrease.
However, the pendulum has not made a full swing away from bypass graft surgery. Isolated severe stenosis of the left anterior descending, as well as multifocal stenoses, is currently being treated with minimally invasive direct coronary artery bypass surgery with results comparable to stenting.4
Types of Bypass Grafts
The most common types of grafts are aortocoronary saphenous vein grafts and left internal mammary grafts, followed by right internal mammary grafts, radial artery grafts, and gastroepiploic artery grafts.
Mammary grafts are generally anastomosed directly to the left anterior descending artery. Vein grafts and radial artery grafts are often sequential, meaning that there are “touchdown” or side-to-side anastomoses along the course of the graft before the terminal anastomosis. Furthermore, vein and radial artery grafts may be inserted proximally into other vein grafts (“Y” conduits), minimizing proximal aortic anastomotic sites.
Graft Failure Rates
Vein grafts show poor long-term patency and limit the long-term success of coronary artery bypass grafting; ˜60% to 70% will be narrowed or closed down within a decade. In contrast, 90% of internal mammary artery grafts remain patent at 10 years.5 A recent 1-year angiographic study showed 100% patency rate for mammary artery grafts compared to only 70% for vein grafts.6
Because of the known increased longevity of mammary grafts over saphenous vein grafts, some centers perform bilateral mammary grafting; however, there has been no demonstrable difference in survival to single left internal mammary grafting.7 Similarly, patients who received multiple grafts to each major diseased artery territory have early outcomes similar to those who received single grafts per territory, and constructing multiple grafts to each major diseased artery territory increases operative time and does not improve long-term survival.8 Radial artery grafts have an intermediate failure rate between saphenous veins and internal mammary arteries, about 30% in 5 years.9
Survival
Long-term survival after coronary artery bypass surgery is generally excellent. Meta-analyses have shown 96% and 85% survival at 1 and 5 years, respectively,10 and 86%, 48%, 19%, and 7% at 5, 15, 25, and 35 years after surgery, respectively.11
There are many factors that affect survival adversely, including multivessel disease, diabetes, prior bypass grafting, preoperative low ejection fraction or myocardial infarct, older age, emergency surgery, mitral incompetence, smoking, respiratory disease, and hypertension.12,13,14
Surgical Techniques
There are several techniques used for cardiac immobilization in order to perform bypass surgery. The classic procedure is cold cardioplegia with extracorporeal bypass. “Off-pump” denotes lack of bypass, with the heart stabilizers (such as the Octopus) used for suturing. In general, completeness of revascularization is more important for survival than technique employed.18 Minimally invasive bypass often uses a robotic system (e.g., the Da Vinci robotic system used for cancer surgeries), with a keyhole opening. Minimally invasive procedures are generally limited to left anterior descending disease with internal mammary grafts, however. Completely robotic surgery via endoscopy is also possible using special bypass procedures. Such procedures are termed total endoscopic coronary artery bypass (TECAB) or endoscopic atraumatic coronary artery bypass (endoACAB).
Currently, the term “minimal invasive direct coronary artery bypass” (MIDCAB) is used to designate coronary bypass procedures that do not require sternotomy, cardiopulmonary bypass, or even blood transfusions.19,20 These procedures employ minimal invasive extracorporeal circulation techniques (off-pump) that have replaced the heart-lung machine and reduced the need for transfusion.19,20
Pathologic Evaluation
Postmortem Radiography
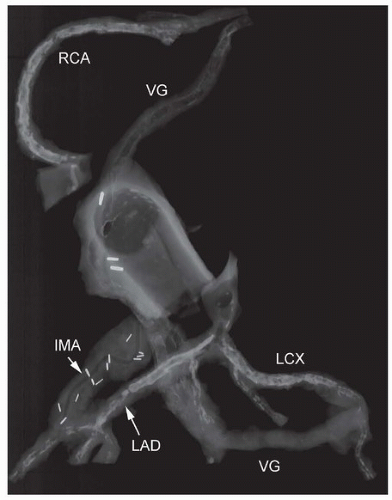
Radiographic assessment is of great benefit in identifying sites of anastomosis, mammary grafts (with multiple surgical clips), and presence of stents, in either native vessels or grafts. In addition, postmortem radiography identifies the degree of coronary and graft calcification and will additionally demonstrate calcification of the aortic valve and mitral annulus (Fig. 154.1).
Postmortem Angiography
Used generally as a research tool, angiography can be performed on fresh autopsy hearts after injection of barium-formalin mixtures. Injection can be done after perfusion fixation at ˜100 mm Hg or into fresh specimens with controlled pressures.21
Dissection Techniques
Gross dissection of bypass grafts is made difficult by pericardial adhesions, which are invariably present. The proximal aorta and pulmonary artery need to be freed of adhesions, such that the proximal trunks can be transected for exposure of the semilunar valves (Figs. 154.2 and 154.3).
The proximal right and left ostia are then identified, and the arteries are removed for decalcification and further radiography, if desired. Sections of grafts themselves, if taken, can be identified by arterial target (e.g., saphenous vein graft to left obtuse marginal, SVG LOM). Sections of anastomotic sites can be identified by graft, vessel, and anastomosis (e.g., IMA LAD ANA). To obtain histologic sections of anastomotic sites, the native artery should be sectioned at cross sections as usual, with three sections: one just proximal to, one at, and one just distal to the anastomosis (Figs. 154.4 and 154.8). Sections of native arteries are especially important for evaluation of early deaths. Runoff vessels should be examined for luminal diameter and degree of atherosclerotic narrowing. Side-to-side anastomoses are harder to document histologically, but an attempt should be made to evaluate degree of stenosis before and after the “touchdown” site (Table 154.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree