Chapter 5 Control of breathing
 The respiratory centre in the medulla generates the respiratory rhythm using an oscillating network of six groups of interconnecting neurones.
The respiratory centre in the medulla generates the respiratory rhythm using an oscillating network of six groups of interconnecting neurones. Many other diverse areas of the central nervous system influence respiratory control, these connections being coordinated by the pons.
Many other diverse areas of the central nervous system influence respiratory control, these connections being coordinated by the pons. Irritant and stretch receptors in the lungs and diaphragm are involved in a series of reflex actions on the respiratory centre to influence respiratory activity.
Irritant and stretch receptors in the lungs and diaphragm are involved in a series of reflex actions on the respiratory centre to influence respiratory activity. Central chemoreceptors respond to changes in pH caused by alterations in carbon dioxide partial pressure, rapidly increasing ventilation in response to elevated arterial Pco2.
Central chemoreceptors respond to changes in pH caused by alterations in carbon dioxide partial pressure, rapidly increasing ventilation in response to elevated arterial Pco2.Early in pregnancy the fetal brainstem develops a ‘respiratory centre’, which produces uninterrupted rhythmic breathing activity for many years.1 Throughout life the subject is mostly unaware of this action, which is closely controlled by a combination of chemical and physical reflexes. In addition, when required, breathing may (within limits) be completely over-ridden by voluntary control or interrupted by swallowing and involuntary non-rhythmic acts such as sneezing, vomiting, hiccupping or coughing. The control system is highly complex, with its automatic ability to adapt the action of the respiratory muscles to the changing demands of posture, speech, voluntary movement, exercise and innumerable other circumstances which alter the respiratory requirement or influence the performance of the respiratory muscles.
The Origin of the Respiratory Rhythm2
Early attempts to find the site of respiratory control used an anatomical approach involving the removal or stimulation of specific areas of the brainstem in animals (page 244). Subsequent development of precise imaging techniques allowed localisation of respiratory regions in normal human subjects, and these studies confirm that much of the historical animal work does apply to humans.3 The anatomical approach to understanding respiratory control has also been succeeded by a biochemical approach as new research methods and the possibility of therapeutic intervention have led to an explosion of interest in the chemical interactions between and within respiratory neurones.
Anatomical Location of the ‘Respiratory Centre’
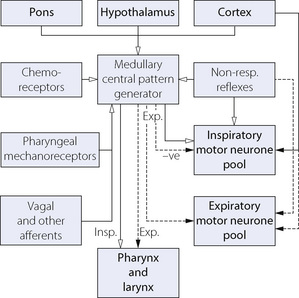
The medulla is accepted as the area of brain where the respiratory pattern is generated and where the various voluntary and involuntary demands on respiratory activity are coordinated. There are many neuronal connections both into and out of the medulla, as summarised in Figure 5.1, the functions of which are described below.
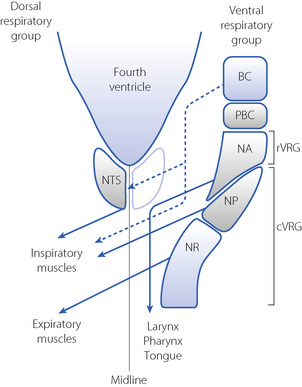
Respiratory neurones in the medulla are mainly concentrated in two anatomical areas, the ventral and dorsal respiratory groups, which have numerous interconnections (Figure 5.2).
The dorsal respiratory group lies in close relation to the nucleus tractus solitarius, where visceral afferents from cranial nerves IX and X terminate (Figure 5.2). It is predominantly composed of inspiratory neurones with upper motor neurones passing to the inspiratory anterior horn cells of the opposite side. The dorsal group is primarily concerned with timing of the respiratory cycle.
The ventral respiratory group comprises a column of respiratory neurones:4
 caudal ventral respiratory group, including the nucleus retroambigualis, which is predominantly expiratory with upper motor neurones passing to the contralateral expiratory muscles, and the mainly inspiratory nucleus para-ambigualis that controls the force of contraction of the contralateral inspiratory muscles.
caudal ventral respiratory group, including the nucleus retroambigualis, which is predominantly expiratory with upper motor neurones passing to the contralateral expiratory muscles, and the mainly inspiratory nucleus para-ambigualis that controls the force of contraction of the contralateral inspiratory muscles. rostral ventral respiratory group, mostly made up of the nucleus ambiguous, which is involved in airway dilator functions of the larynx, pharynx and tongue.
rostral ventral respiratory group, mostly made up of the nucleus ambiguous, which is involved in airway dilator functions of the larynx, pharynx and tongue. Pre-Bötzinger complex, believed to be the anatomical location of the central pattern generator (CPG).
Pre-Bötzinger complex, believed to be the anatomical location of the central pattern generator (CPG).Central Pattern Generator2,5
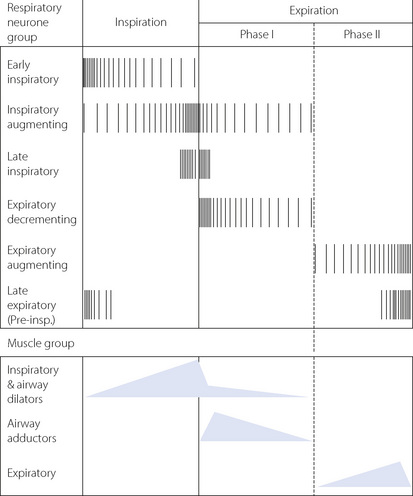
Unlike in the heart, there is no single ‘pacemaker’ neurone responsible for initiating breathing. Instead, a group-pacemaker hypothesis is proposed in which groups of associated neurones generate regular bursts of neuronal activity.6 For breathing, the group-pacemaker involves a complex interaction of at least six groups of neurones with identifiable firing patterns spread throughout the medulla, though concentrated in the region of the pre-Bötzinger complex. Groups of neurones include early inspiratory, inspiratory augmenting (Iaug), late-inspiratory interneurones (putative ‘off-switch’ neurones), early expiratory decrementing, expiratory augmenting, and late expiratory pre-inspiratory neurones. Typical firing patterns and the resulting muscle group activity are shown schematically in Figure 5.3. The resultant respiratory cycle may be divided into three phases:
In practice, many such rhythm-generating networks are represented in parallel, so it is difficult to abolish the respiratory rhythm even with extensive brainstem damage.
Cellular mechanisms of central pattern generation.2 Respiratory neurones that exhibit spontaneous activity achieve this by a combination of intrinsic membrane properties and excitatory and inhibitory feedback mechanisms requiring neurotransmitters. In practice, neurotransmitters (both inhibitory and excitatory) have a dual effect – they recruit other cells by direct activation and modulate the spontaneous activity of a single cell by effects on its own membrane ion channels, for example slowing the rate at which an action potential travels along a dendrite.
In a similar fashion to rhythm generation in cardiac tissue, a combination of potassium and calcium ion channels are involved. For instance, in a single Iaug neurone slow membrane depolarisation occurs so producing a spontaneous discharge. These cells then ‘recruit’ other Iaug cells by excitatory postsynaptic potentials (EPSPs) and a crescendo of Iaug activity develops. Calcium-dependent potassium channels then begin to be activated and repolarise the cells so ‘switching off’ the Iaug respiratory group. Activation of other cell groups, for instance expiratory augmenting neurones, will result in activation of inhibitory postsynaptic potentials (IPSPs) on the Iaug neurones to hyperpolarise the neurone and inhibit the next wave of inspiratory activity. Similar membrane effects occur in all the respiratory neurone groups shown in Figure 5.3.
Neurotransmitters Involved in CPG and Respiratory Control2,7
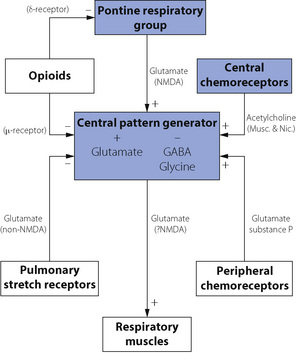
These are summarised in Figure 5.4. Central pattern generation requires a combination of excitatory and inhibitory neurotransmitters. Excitatory amino acids (usually glutamate) activate several different receptors. These are divided into two groups; N-methyl-d-aspartate (NMDA) receptors, which are fast acting ion channels and non-NMDA receptors, which are slower reacting receptors involving G-protein mediated effects. Inhibitory neurotransmitters include glycine and γ-aminobutyric acid (GABA) acting via specific glycine receptors and GABAA receptors respectively to hyperpolarise the neurone and thereby inhibit its activity. These two inhibitory transmitters act quite independently during different phases of CPG.
Neuromodulators are substances that can influence the CPG output, but are not themselves involved in rhythm generation. There are numerous neuromodulators of respiration, many of which have several subtypes of receptors. Their exact role in normal human respiration remains unclear, but they are of undoubted relevance in both normal and abnormal breathing. For example, exogenous opioids are known to have a profound depressant effect on respiratory activity in humans (page 77), indicating the presence of opioid receptors in the respiratory centre, but administration of the opioid antagonist naloxone has no effect on respiration in resting normal subjects. Other neuromodulators include acetylcholine, which acts via both muscarinic and nicotinic receptors to mediate the effect of central chemoreceptors on respiration. Serotonin (5-hydroxytryptamine, 5HT) has many conflicting effects on respiration as a result of the numerous receptor subtypes present. Glutamate acts as a neuromodulator via both NMDA and non-NMDA receptors to mediate the pontine influence on CPG and is also involved in the influence of pulmonary stretch receptors and peripheral chemoreceptors on the respiratory pattern. Substance P also has an excitatory influence resulting in an increase in tidal volume in response to peripheral chemoreceptor activity. This diverse collection of neuromodulators probably all ultimately act via a common intra-cellular signalling pathway within CPG neurones, involving protein kinases A and C that in turn influence the activity of GABA, glycine and glutamate linked potassium and chloride channels.8
Efferent Pathways from the Respiratory Centre
Respiratory motor neurones in the brainstem are pooled into two separate areas, corresponding to inspiratory and expiratory muscle activity (see Figure 5.1). The complex integration of respiratory control seen in the CPG neurones continues to take place at the junction of the upper motor neurone with the anterior horn cell of the lower motor neurone. Three groups of upper motor neurones converge on the anterior horn cells supplying the respiratory muscles. The first group of upper motor neurones is from the dorsal and ventral respiratory groups of the medulla and are concerned with both inspiratory and expiratory output from the CPG. The second group is concerned with voluntary control of breathing (speech, respiratory gymnastics, etc.) and the third group with involuntary non-rhythmic respiratory control (swallowing, cough, hiccup, etc.). Each group of upper motor neurones occupies a specific anatomical location within the spinal cord. Neuronal control of the respiratory muscles is described in Chapter 6.
Central Nervous System Connections to the Respiratory Centre
Cerebral Cortex9
Breathing can be voluntarily interrupted and the pattern of respiratory movements altered within limits determined mainly by changes in arterial blood gas tensions. This is essential for such acts as speech, singing, sniffing, coughing, expulsive efforts and the performance of tests of ventilatory function. The neurones involved in this cortical ‘over-ride’ of respiration may completely bypass the respiratory centre and act directly on the respiratory muscle lower motoneurones.10
Volitional changes in respiration are common, and under some circumstances overcome the usual chemical control of respiration. For example, conscious respiratory drive may well maintain breathing in subjects following voluntary hyperventilation when the Pco2 is below the apnoeic threshold (page 70). There are usually minor changes in the respiratory pattern when subjects focus their attention on their breathing as when physiological mouth pieces or breathing masks are used.11
In addition to volitional changes in the pattern of breathing, there are numerous other suprapontine reflex interferences with respiration such as sneezing, mastication, swallowing and coughing.12 Reflex control of respiration during speech is complex.13 During prolonged conversation, respiratory rate and tidal volume must be maintained approximately normal to prevent biochemical disturbance. In addition, for speech to be easily understood, pauses to allow inspiration must occur at appropriate boundaries in the text – for example between sentences. To achieve this, the brain performs complex assessments of the forthcoming speech to select appropriate size breaths to prevent cumbersome interruptions. This is easier to achieve during reading aloud when 88% of breaths are taken at appropriate boundaries in the text, compared with a figure of only 63% during spontaneous speech.13
Ondine’s Curse (Primary Alveolar Hypoventilation Syndrome)
In 1962 Severinghaus & Mitchell14 described three patients who exhibited long periods of apnoea, even when awake, but who breathed on command. They termed the condition ‘Ondine’s curse’ from its first description in German legend. The water nymph, Ondine, having been jilted by her mortal husband, took from him all automatic functions, requiring him to remember to breathe. When he finally fell asleep, he died. The condition is seen in adults with primary alveolar hypoventilation occurring as a feature of many different diseases, including chronic poliomyelitis and stroke.15 Characteristics include a raised Pco2 in the absence of pulmonary pathology, a flat CO2/ventilation response curve and periods of apnoea which may be central or obstructive. A similar condition is also produced by overdosage with opioids.
Ondine’s curse is also used to describe the rare condition of congenital central hypoventilation syndrome in which babies are born with a permanent defect in automatic respiratory control, leading to apnoea and hypoventilation during sleep.16 In addition, these children have abnormal respiratory responses to exercise and in keeping with the German legend also have abnormalities of cardiac control.17 In spite of such severe abnormalities, non-invasive methods of nocturnal ventilation and diaphragmatic pacing have led to almost normal lives for many of these children.16
Peripheral Input to the Respiratory Centre and Non-Chemical Reflexes
Reflexes Arising from the Upper Respiratory Tract18,19
Nose. Water and stimulants such as ammonia or cigarette smoke may cause apnoea as part of the diving reflex (page 313). Irritants can initiate sneezing which, unlike coughing, cannot be undertaken voluntarily.
Pharynx. Mechanoreceptors that respond to pressure play a major role in activation of the pharyngeal dilator muscles (page 83). There is ample evidence that local anaesthesia of the pharynx impairs their action. Irritants may cause bronchodilatation, hypertension, tachycardia, and secretion of mucus in the lower airway.
Larynx. The larynx has a dense sensory innervation with fibres from the subglottic region in the recurrent laryngeal nerve and those from the supraglottic region in the internal branch of the superior laryngeal nerve. Most reflexes arise from the supraglottic area, as section of the latter nerve abolishes almost all reflex activity. There are three groups of receptors. Mechanoreceptors respond to changes in transmural pressure or laryngeal motion and result in increased pharyngeal dilator muscle activity, particularly during airway obstruction. Cold receptors are found superficially on the vocal folds and activation generally results in depression of ventilation. The importance of this reflex in adult humans is uncertain, but these receptors may also produce bronchoconstriction in susceptible individuals (Chapter 28). Irritant receptors respond to many substances such as distilled water, cigarette smoke and inhaled anaesthetics, and, in a similar fashion to direct mechanical stimulation of the larynx, cause cough, laryngeal closure and bronchoconstriction.
The cough reflex.20 This may be elicited by chemical or mechanical stimuli arising in the larynx, trachea, carina or main bronchi. Which of these sites is responsible for the initiation of a cough is difficult to determine. For chemical stimuli the larynx may be of less importance as superior laryngeal nerve block has little effect on cough stimulated by citric acid inhalation,21 and in patients following heart–lung transplant inhalation of the normally potent stimulant distilled water results in little or no cough (page 505). Coughing can be initiated or partially inhibited voluntarily but the reflex is complex and comprises three main stages:
Expiration reflex.20,22 Similar to a cough, this reflex originates in the larynx and is believed to exist in order to prevent material being aspirated into the upper airway. It differs from a cough by the absence of an inspiratory phase, the compressive and expulsive phases occurring immediately and from the lung volume present at the time the larynx is irritated. The distinction between the cough and expiration reflexes is important – a large inspiration as seen at the start of a cough would not be helpful in the presence of solid or liquid at the laryngeal inlet.
Reflexes Arising in the Lung
Pulmonary stretch receptors and their associated reflexes.23 There are many different types of receptors in the lungs sensitive to inflation, deflation, mechanical and chemical stimulation, afferents from which are mostly conducted by the vagus, although some fibres may be carried in the sympathetic nerves. Slowly adapting stretch receptors (SARs) are found predominantly in the airways rather than in the alveoli, and are closely associated with the tracheo-bronchial smooth muscle. Lung inflation stimulates the SARs, which are named ‘slowly adapting’ due to their ability to maintain their firing rate when lung inflation is maintained, thus acting as a form of lung volume sensor. Conversely, rapidly adapting stretch receptors (RARs) are located in the superficial mucosal layer,18 and are stimulated by changes in tidal volume, respiratory frequency or changes in lung compliance.23 The RARs also differ from SARs in being nociceptive and chemosensitive, responding to a wide range of chemical irritants, mechanical stimuli and inflammatory mediators.
How these receptors transduce a mechanical change in the tissue into an action potential is unknown. Hypotheses include the release of mediators from nearby associated cells that activate a receptor on the neurone, or ion channels may exist that respond directly to an alteration in their physical shape.24 Afferent nerves from all these receptors converge on the nucleus tractus solitarius (NTS) of the medulla, where their signals are modulated and coordinated before further polysynaptic pathways communicate with the other regions of the respiratory centre. This processing of the afferent inputs by the NTS is believed to be capable of neuronal plasticity, which means the modulation can be altered by prolonged changes in external environment that influence the afferent inputs.25
The reflexes associated with pulmonary stretch receptors have attracted much attention since the associated inflation and deflation reflexes were described by Hering & Breuer in 1868.26 Breuer was a clinical assistant to Professor Hering but apparently the work was at his own instigation. However, Hering, who was a corresponding member of the Vienna Academy of Science, published Breuer’s work under his own name, in accord with the custom of the time. Breuer’s role was clearly stated in Hering’s paper but he was not a co-author. Later the same year, Breuer published a much fuller account of his work under his own name.
The significance of the Hering–Breuer reflex in humans is controversial.27,28 There appears to be an important species difference between laboratory animals, in which the reflex is easy to demonstrate, and humans in whom the reflex is very weak.29 Pragmatic evidence that the Hering–Breuer reflex is unimportant in awake humans comes from studies showing normal breathing patterns in volunteers following bilateral vagal nerve block30 and in patients who have had bilateral lung transplants, when both lungs must be totally denervated (Chapter 33). Conversely, studies in which conscious perception of chest wall position is suppressed by applying imperceptible amounts of assisted ventilation have demonstrated that respiratory pattern is altered within the physiological range, demonstrating the presence of a vagal feedback mechanism.28,31 Although the Hering–Breuer inflation reflex therefore appears to exist but have minimal functional significance in adults, it is widely accepted as being present in neonates and infants.32
The deflation reflex consists of an augmentation of inspiration in response to deflation of the lung and can be demonstrated in man.33 These results are consistent with the hypothesis that lung deflation has a reflex excitatory effect on breathing, but that the threshold is higher in man than for other mammalian species.
Head’s paradoxical reflex. Head, working in Professor Hering’s laboratory, described a reversal of the inflation reflex.34 Many authors have reported that, with normal vagal conduction, sudden inflation of the lungs of many species may cause a transient inspiratory effort before the onset of apnoea due to the inflation reflex.29 A similar response may also be elicited in new-born infants,35 but it has not been established whether this ‘gasp reflex’ is analogous to Head’s paradoxical reflex. All anaesthetists are aware that, after administration of respiratory depressants, transient increases in airway pressure often cause an immediate deep gasping type of inspiration.
Other Pulmonary Afferents
C-fibre endings lie in close relationship to the capillaries, one group is in relation to the bronchial circulation and the other to the pulmonary microcirculation. The latter correspond to Paintal’s juxta-pulmonary capillary receptors (J receptors, for short).18,36
These receptors are relatively silent during normal breathing but are stimulated under various pathological conditions. They are similar to RARs described above, being nociceptive and activated by reactive oxygen species,37 tissue damage, accumulation of interstitial fluid and release of various mediators. In the laboratory they can be activated by intravascular injection of capsaicin to produce the so-called pulmonary chemoreflex which comprises bradycardia, hypotension, apnoea or shallow breathing, bronchoconstriction and increased mucus secretion.18,28 They may well be concerned in the dyspnoea of pulmonary vascular congestion and the ventilatory response to exercise and pulmonary embolisation. C-fibre endings have been characterised in physiological studies but have never been identified histologically, although non-myelinated nerve fibres are seen in the alveolar walls.
Reflexes Arising from Outside the Airway and Lungs
Phrenic nerve afferents.38 Approximately one-third of neurones in the phrenic nerve are afferent, with the majority arising from muscle spindles and tendon organs forming the spinal reflex arc described on page 89. However, some afferent neurones continue through the ipsilateral spinal cord to the brainstem and somatosensory cortex. Experimental stimulation of phrenic afferent fibres results in a reduction of respiratory efferent activity, but stimulation of some smaller afferent fibres has the opposite effect. Thus the physiological role of phrenic afferents remains obscure, but it is unlikely that they have any influence on normal breathing. The sensory information provided by phrenic afferents is believed to be important in the perception of, and compensation for, increased inspiratory loads and these afferents are important in the ‘breaking point’ following a breath hold (page 76).
Afferents from the musculoskeletal system. These probably do not contribute to normal resting ventilation but have an important role in the hyperventilation of exercise (Chapter 15).
The Influence of Carbon Dioxide on Respiratory Control39,40
For many years it was believed that the respiratory centre itself was sensitive to carbon dioxide. However, it is now known that both central and peripheral chemoreceptors are responsible for the effect of carbon dioxide on breathing, the latter accounting for about 80% of the total ventilatory response.39 Because of their reliance on extra-cellular pH (see below) the central chemoreceptors are regarded as monitors of steady-state arterial Pco2 and tissue perfusion in the brain, while the peripheral chemoreceptors respond more to short term and rapid changes in arterial Pco2.41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree