9 Contrast Perfusion Echocardiography
Background/Basic Principles
Ultrasound Contrast Agents
• The currently available ultrasound contrast agents (UCAs) in the United States are Optison (General Electric, Waukesha, WI) and Definity (Lantheus, N. Billerica, MA). In Europe, Sonovue (Bracco, Milan, IT) is an approved contrast agent.
• Contrast agents in the United States are currently approved only for left ventricular (LV) opacification. However, these agents can also be utilized to examine myocardial perfusion with perfusion imaging techniques (available on Philips [Andover, MA] and Siemens [Mountain View, CA] ultrasound scanners).
• Perfusion imaging adds incremental value to resting wall motion assessment. Following myocardial infarction, myocardial contrast enhancement within the risk area predicts recovery of function, independent of what resting wall motion is. In the evaluation of chronic coronary artery disease (CAD), the assessment of resting myocardial perfusion is also predictive of recovery of function following revascularization. Perfusion imaging adds incremental value to stress wall motion assessment. Perfusion imaging during dobutamine or exercise stress echo- cardiography detects abnormalities that antedate wall motion abnormalities and helps delineate subendocardial wall thickening abnormalities that are induced even when overall transmural wall thickening appears normal. Perfusion imaging may also help identify vascular masses.
Approaches to Myocardial Perfusion Imaging
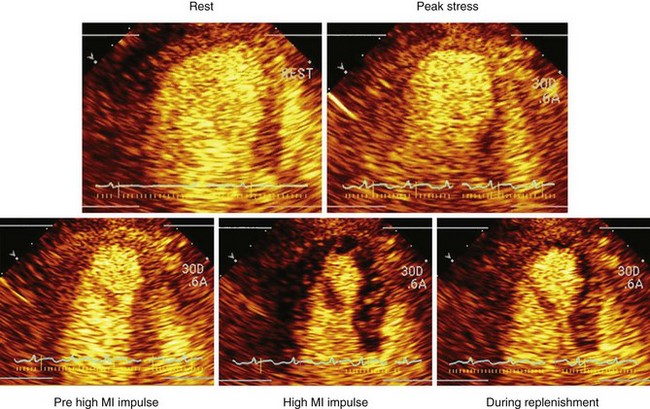
• Myocardial perfusion is typically examined during an infusion of microbubbles, during which a high mechanical index (MI) impulse (referred to as flash) is delivered to clear the capillaries of microbubbles. The rate of replenishment of myocardial contrast, and the plateau intensity, are subsequently examined either visually or quantitatively (Fig. 9-1).
• RTPE is a low-MI imaging technique that permits the real-time detection of myocardial contrast enhancement following either a small (0.1–0.2 mL) bolus injection or continuous infusion of UCA. Alternatively, since continuous higher MI imaging destroys microbubbles, myocardial perfusion may be assessed with high-MI imaging at triggered intervals (end-systolic frames), where contrast enhancement is visualized at progressively longer intervals after the bubbles have been destroyed by an initial high-MI impulse. The advantages and disadvantages of triggered perfusion imaging versus RTPE are outlined in Table 9-1.
TABLE 9-1 ADVANTAGES AND DISADVANTAGES OF LOW–MECHANICAL INDEX REAL-TIME PERFUSION ECHOCARDIOGRAPHY VERSUS HIGH–MECHANICAL INDEX TRIGGERED PERFUSION IMAGING
| RTPE | Triggered Perfusion Imaging | |
|---|---|---|
| Frame rate | 20–25 Hz | Triggered end-systolic at incremental intervals |
| Sensitivity | Good | Excellent |
| Dynamic range | Low | High |
| Utility during exercise stress | Yes | Not feasible |
| Utility during dobutamine stress | Yes | Not feasible |
| Utility during vasodilator stress | Good | Excellent |
| Utility during resting perfusion assessment | Excellent | Excellent |
| Simultaneous analysis of wall motion/perfusion | Yes | No |
Physiologic Basis for Examining Myocardial Perfusion with Ultrasound Contrast Agents
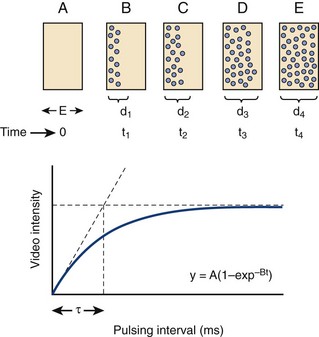
• Changes in myocardial blood flow can be analyzed by examining the replenishment of myocardial contrast following a high-MI impulse. This concept was developed by Wei et al.1 and is demonstrated in Figure 9-2.
• The product of the rate of contrast replenishment (reflecting myocardial red blood cell velocity) and the plateau intensity (reflecting capillary cross sectional area) correlates with myocardial blood flow. By normalizing plateau intensity to adjacent LV cavity intensity, one can compute absolute myocardial blood flow.
• Most clinical applications analyze myocardial perfusion visually. A key concept is that under resting conditions with a typical diagnostic transducer having a 5-mm elevation plane, normal myocardial contrast replenishment should be within 5 seconds, while under hyperemic conditions (exercise, dobutamine, vasodilator stress), replenishment should be within 2 seconds following the high-MI impulse.
Role of Physician
• The physician is responsible for the overall quality control of the procedure, which begins by ensuring that all personnel (cardiology fellows, nurses, sonographers) are adequately educated in the concepts of myocardial perfusion assessment with a continuous infusion of microbubble contrast. The physician needs to work with the lead sonographer and nursing team to develop a standard operating procedure to be followed whenever contrast is utilized to assess perfusion.
• Although RTPE during stress can be performed in a manner similar to standard stress procedures without contrast, the physician assigned to the laboratory needs to be Level III trained in echocardiography and have been trained in the performance and interpretation of at least 50 RTPE examinations before operating independently.
Roles of the Sonographer and Nurse
• Contrast is used to enhance images, improve border detection, and provide information on cardiac perfusion at rest and during functional stress echocardiographic studies.
• The contrast is administered by a registered nurse or other qualified medical personnel. A continuous infusion of Definity or Optison contrast is used both for resting, exercise, vasodilator, and dobutamine stress echocardiograms.
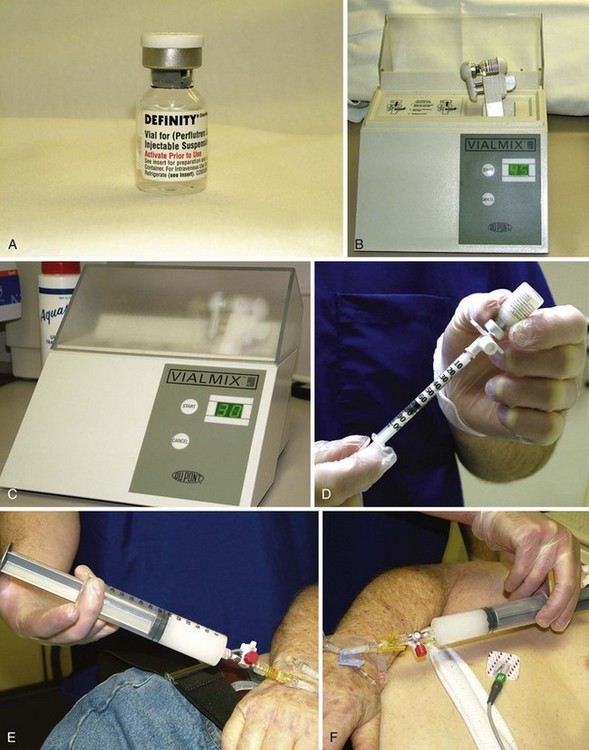
• Definity is activated by agitating the vial for 45 seconds in the Vial Mix (Lantheus, Billerica, MA) (Fig. 9-3).
• Dilute 0.8 mL of Definity into 29 mL normal saline to make a total of approximately 30 mL, as demonstrated in Figure 9-3. One half of a vial of Optison can be diluted into 20 mL of saline if that is the agent desired.
• Do not mix until just prior to infusion. Two syringes should be made for stress echocardiograms. One syringe should be sufficient for resting echocardiograms. The infusion rate is approximately 4 mL/min by hand, watching the clock.
• The solution should be mixed back and forth periodically so that the contrast does not settle in the bottom of the syringe.
• The infusion is started when the sonographer is ready to capture resting images. The nurse will start the infusion and the sonographer will start to acquire the resting images.
• The infusion rate starts at 4 mL/min and can be increased or decreased if needed; this is dependent on the images. The sonographer will inform the nurse to increase or decrease the infusion rate as needed.
• One syringe of the mixture should be sufficient for a resting echocardiogram when looking for perfusion, LV function, wall motion abnormalities, and overall ejection fraction. This is also usually sufficient when examining for intracavitary thrombi or vascular tumors.
• The second half of the ultrasound contrast will be needed both for exercise and pharmacologic stress echocardiograms. In addition, any leftover diluted contrast from the syringe used to obtain resting images can be used for stress images as well. The range of solution left over from the resting images is from 0 to 20 mL for Definity, depending on the difficulty in obtaining the resting images.
• The second syringe is used for the stress images. In this setting, the infusion rate is usually lower because of the higher cardiac output (2–4 mL/min). The infusion rate should remain constant unless the sonographer indicates otherwise. It is important not to mix the second syringe until the sonographer is ready to capture the intermediate images for dobutamine stress echocardiography (DSE) and immediate post-stress images for exercise stress echocardiography.
Advantages and Disadvantages of Using Real-time Perfusion Echocardiography versus Other Imaging Techniques
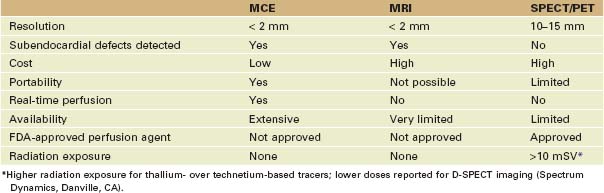
• Ultrasound has higher spatial resolution than either radionuclide imaging (single-photon emission computed tomography [SPECT] or positron emission tomography [PET]) techniques and provides greater temporal resolution. This permits the detection of myocardial perfusion in real time along with regional function assessments. Despite this, SPECT and PET are the only techniques approved by the U.S. Food and Drug Administration (FDA) for examining myocardial perfusion.
• Myocardial perfusion imaging is also possible with magnetic resonance imaging (MRI). Although the spatial resolution of MRI is comparable with ultrasound, it is not currently capable of measuring myocardial blood flow and blood flow changes as is myocardial contrast echocardiography (MCE) (Table 9-2).
Acquisition of Perfusion Images
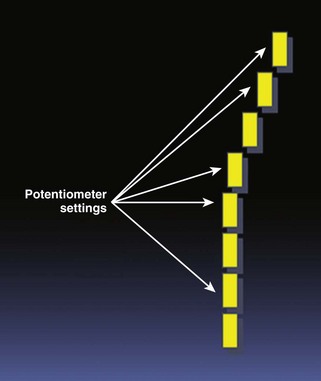
• The infusion rate must be adjusted so as to permit homogeneous myocardial opacification prior to application of high-MI impulses and analysis of contrast replenishment. This will usually require that TGC potentiometers be set to slightly higher in the near field to overcome automatic reductions that typically occur in the near field (Fig. 9-4).
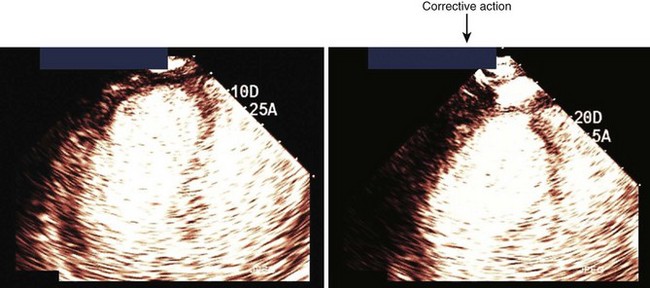
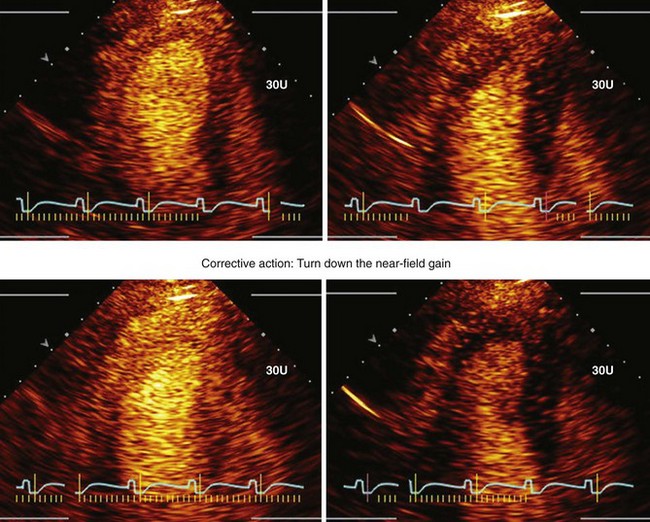
• These settings must be such that a brief high-MI impulse (1.0–1.3 MI) results in clearance of signals from the myocardium. Figure 9-5 demonstrates how to adjust the TGCs to create homogeneous opacification. Figure 9-6 illustrates that occasionally the near-field TGCs must be adjusted back slightly to ensure that the post-high-MI impulse adequately clears the myocardium in all segments.
• The infusion rate of UCA must be adjusted so that the high-MI impulse only clears myocardial contrast and does not excessively destroy LV cavity contrast. This will result in a reduction of contrast entering the coronary circulation and create difficulties in analyzing myocardial contrast replenishment.
• Tissue Contrast Enhancement is available on the Philips iE33 and Sonos 5500/7500. Real-time low-MI imaging is made possible with power modulation. This feature prolongs the contrast effect and provides real-time contrast visualization of perfusion using a low MI (<0.2). Therefore, both wall motion and myocardial contrast can be analyzed simultaneously.
• Cadence Contrast Pulse Sequencing technology is available on the Siemens Acuson Sequoia. This imaging technology provides low-MI imaging with tissue only, contrast only, or both. It recognizes and processes the unique nonlinear fundamental and higher order harmonic signals that are generated by the UCA. The nonlinear response is a result of the bubbles’ asymmetric expansion and contraction with an ultrasound pulse. The contrast signal is significantly increased with this new pulse sequencing scheme.
• Table 9-3 provides the recommended ultrasound settings and presets for both the Philips iE33 and the Acuson Sequoia™ when using RTPE.
• Low-MI contrast imaging (or RTPE) is dependent on the microbubbles resonating without disruption in the LV. The correct system adjustments are needed in order to have adequate bubble concentration in the LV cavity. Table 9-4 gives the definitions and concepts behind optimal settings for real-time perfusion imaging.
TABLE 9-3 IMAGING SETTINGS/PRESETS FOR REAL-TIME PERFUSION IMAGING
| Presets for Philips iE33 | Presets for Acuson Sequoia | |
|---|---|---|
| Depth | 140 mm | 140 mm |
| Focus | Mitral valve level | Mitral valve level |
| Contrast option | Gen (1.5 MHz) | CPS (Cadence contrast agent imaging) |
| Gain/compression | 60-68/50 | CPS gain: −8 to −15 |
| High-MI flash frame duration | 2–5 frames at rest 5–20 frames during stress | 2–5 frames at rest; 1–2 heartbeats during stress |