Fig. 39.1
Projected characteristics of infant and child size pumps based on the adult Jarvik 2000
There were also a number of practical manufacturing considerations including the high precision required to maintain very close tip clearances in the axial pump designs. Some parts must have very thin wall sections. Machining methods and quality assurance were critical to success.
The approach we used was to calculate motor power requirements based on reasonable assumptions about pump and motor size and efficiency, design the motor for peak efficiency at the nominal pump operating point, and seek to maximize motor efficiency within the physical size constraints of the planned device layout and available battery voltage. Only last after most of the physical dimensions of the device had been determined, did we optimize the blade shapes. Our approach to optimization of the blade shapes for the proposed pediatric pumps utilized CFD methods much more than we had done previously with the adult model. The initial blade designs were based on two-dimensional theory, but also used three-dimensional CAD modeling of the blade and flow channel shapes and CFD modeling to identify high shear regions, areas of potential stasis and thrombus formation, and to predict pump hemolysis.
Using data available from the adult model Jarvik 2000, and the many small pumps we have tested, we made initial calculations to establish the sizes of the child and infant models. Major hydrodynamic parameters that determine pump flow and pressure including the rotor hub and tip velocity and the cross-sectional area of the flow path through the blade system were selected. The child and infant models keep the hub and tip velocity almost the same as with the adult model and reduced the cross-sectional flow area in proportion to the lower flow requirements. This established the physical dimensions of the motor, by establishing the motor ID and OD and the motor air gap dimension through which the blood flows. The speed and torque requirements of the motor are determined by the required impeller tip velocity and the required output power of the motor. With these values known, the motors were designed using computer modeling techniques.
The infant pump that we developed was 11 mm diameter, small enough to fit a newborn, but this proved to be too small to achieve enough flow for infants over 10 kg, unless speed was increased to 32,000 rpm (◘ Fig. 39.2).
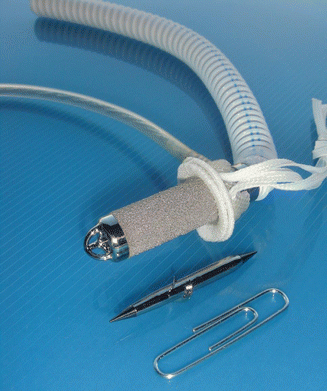

Fig. 39.2
Infant Jarvik 11 mm
We submitted our animal data together with extensive other design and validation data for the infant Jarvik 2000 system to the FDA. The agency informed us that the hemolysis that occurred was unacceptably high, and the condition of the in vivo animals was not good enough for approval. At that speed the pump produced excessive hemolysis, so after extensive efforts to lower hemolysis using redesigned pump blades, we abandoned the 11 mm design.
We decided to optimize the design with CFD studies done by Dr. Jingchun Wu of Advanced Design Optimization, LLC. Jarvik Heart provided a 3D CAD model of the 11 mm pump and Dr. Wu analyzed it (◘ Fig. 39.3).
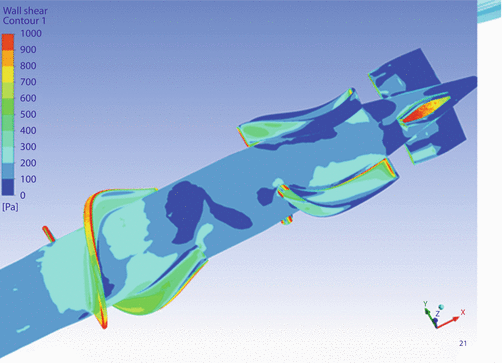

Fig. 39.3
The model predicted that the highest shear would occur at the blade tips and gaps between stationary and rotating components
We postulated that much of the hemolysis might be originating from the bearings at the high speeds, which were needed to obtain sufficient flow and pressure from the 11 mm design. We fabricated pumps that had no impeller blades and no stator blades. Thus the only sites of high shear that remained were the gaps between the rotating conical ceramic bearing shaft and the tips of the supporting posts. Hemolysis caused by the bearings increased substantially at speeds above 18,000 rpm and at 30,000 rpm would be too high as confirmed by normalized index of hemolysis (NIH) and in vivo experiments.
To keep the speed as low as possible, we decided to increase the diameter of the pump from 11 to 15 mm, named infant Jarvik 2015 (◘ Fig. 39.4a, b). This proved to be a major improvement.


Fig. 39.4
a Sectioned view of the infant Jarvik 2015 blood pump with adjustable outflow elbow. b The final design infant Jarvik 2015
Increasing the diameter of the pump increases the cross-sectional area of the flow path, reduces resistance, and increases flow. Increasing the diameter also increases the tip speed at a given rotational speed, which allows the pump to operate at a lower speed for a given pressure. Lower speed permits the pump to avoid blood damage caused by high shear at the bearings. The new pump gave an excellent flow from .5 to 3 L/min at a maximum speed of 18,000 rpm with an NIH (normalized index of hemolysis) <.05 g/100 L and good pressure/flow curves (◘ Fig. 39.5) and proper power consumption (◘ Fig. 39.6).
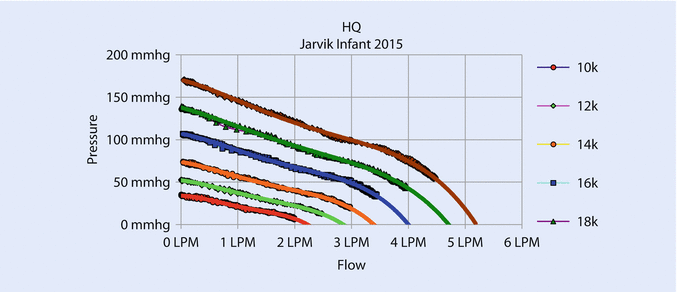
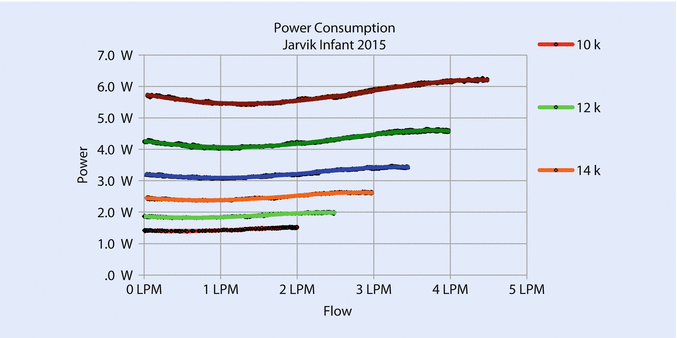

Fig. 39.5
Flow/pressure curves show that the device can pump up to 3 L/min at 18,000 rpm

Fig. 39.6
Power consumption remains below 5 watts for speeds up to 18,000 rpm
In vivo tests are now under way with the infant Jarvik 2015. To date we have conducted three non-GLP implants in 20–30 kg sheep, sacrificed at 30 days. The animals showed low hemolysis.
Additionally we have begun a series of GLP animals for 60-day survival. Since these animal experiments are still under way at the time of writing this chapter, details are unavailable, but in general the hemolysis is low, and the animal’s condition has been excellent except in two cases of surgical problems unrelated to the pump.
Plasma Hb values were mostly below 5–10 mg/dl. There was no thrombus in the pumps or grafts. The kidneys and other organs were free of infarcts (◘ Fig. 39.7). Most animals were healthy, appeared normal, and were free of any serious pump-related problems. Lab values were also essentially normal and unremarkable.
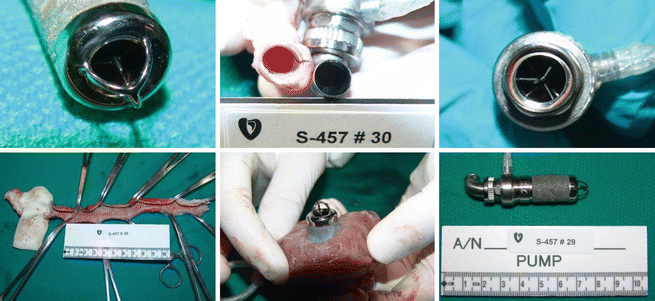

Fig. 39.7
Clockwise from upper left: clean inflow cage and bearing, clean outflow elbow, clean outflow bearing, clean outflow graft, microsphere housing well healed to the endocardial tissue, pump removed from the heart. This pump is typical of all of the infant Jarvik 2015 pumps implanted up to 60 days
FDA approval is still ongoing and the procedure will be over at the end of August 2016. Once the FDA approval will be gained, the clinical trial will be started within 2016.
Clinical Experience
The infant Jarvik pump (11 mm) was used at Bambino Gesù Hospital in Rome for a compassionate use in a 1-year-old male affected by an idiopathic dilated cardiomyopathy in 2012. Patient was preliminary treated implanting a 10 ml Berlin Heart EXCOR LVAD when the patient was 11 months old with a weight of 5 kg. Patient was supported for 123 days. Finally, he experienced severe Berlin Heart EXCOR cannula infection leading to mediastinitis and requiring the device explantation. He was on LVAD (Levitronix) from the left atrium to the ascending aorta for 25 days with sternum open and mediastinal irrigation. Therefore, for compassionate use, the patient was treated implanting an infant Jarvik pump (◘ Fig. 39.8). After 13 days during battery exchange, the pump stopped, for a pump electric blackout, and it was back on ECMO. In the meantime the patient recovered from the infection, and 7 days later it was possible to implant another 10 ml Berlin Heart EXCOR LVAD. After 20 days, the patient underwent successfully Heart Transplantation, and he is currently alive 4 years after heart transplant.




