Ticagrelor has greater antiplatelet activity than clopidogrel and is approved for use in patients with acute coronary syndrome (ACS). There are limited data on use of ticagrelor in real-world practice. We assessed ticagrelor use in 64,600 patients who underwent percutaneous coronary intervention from January 2012 to March 2014 at 47 Michigan hospitals in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Preprocedural risk of major adverse events was estimated with Blue Cross Blue Shield of Michigan Cardiovascular Consortium risk prediction models. The proportion of patients receiving clopidogrel, prasugrel, and ticagrelor was 72% (n = 46,864), 20% (n = 12,596), and 8% (n = 5,140), respectively, using ticagrelor increasing over time. Ticagrelor was used at 45 hospitals, ranging from 0.5% to 64.9% of discharges. Patients receiving ticagrelor were older (63.6 vs 59.4), more often women (32.9% vs 26.7%), and were more likely to present with ST-segment elevation myocardial infarction (24.4% vs 18.8%), cardiogenic shock within 24 hours (1.3% vs 0.9%), and anginal class IV (47.8% vs 43.0%) (p <0.05). Compared with prasugrel, ticagrelor was prescribed in patients with a higher predicted risk of percutaneous coronary intervention complications: contrast nephropathy (2.5% vs 1.6%), transfusion (2.2% vs 1.4%), and death (1.2% vs 0.7%) (p <0.001); >10% of patients were given prasugrel or ticagrelor for a non-ACS indication. Ticagrelor is prescribed to a higher risk population, and 1 in 10 patients prescribed ticagrelor or prasugrel did not have ACS.
Ticagrelor is the most recent P2Y12 inhibitor approved by the Food and Drug Administration in July 2011 for patients with acute coronary syndrome (ACS) and has now been incorporated into the American College of Cardiology/American Heart Association guidelines for percutaneous coronary intervention (PCI) in ACS. Relative to clopidogrel, ticagrelor offers more rapid onset of action and more pronounced platelet inhibition. In patients who underwent PCI for ACS, compared with clopidogrel, ticagrelor not only offers a mortality benefit and superior anti-ischemic efficacy but also higher risk of bleeding complications. The benefits of ticagrelor in stable coronary artery disease have not yet been established but are currently being explored in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared with Placebo on a Background of Aspirin trial. Previous data suggested that prasugrel, another potent P2Y12 inhibitor that was approved by the Food and Drug Administration in 2009, was commonly being prescribed in patients contrary to the drug label. There are limited data evaluating use of ticagrelor in contemporary practice. The purpose of this study was to evaluate the contemporary use of ticagrelor in patients who underwent PCI at 44 Michigan Hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2).
Methods
The BMC2-PCI is a prospective, multicenter statewide registry of consecutive PCIs and includes all nonfederal PCI hospitals in Michigan. This registry, launched since 1997, represents a regional collaborative effort to assess and improve quality of care and outcomes of patients with coronary disease who undergo PCI. This database has been used for risk assessment, for feedback on outcomes, and for quality improvement initiatives. The institutional review boards of the participating hospitals have either approved or waived the need for approval for participation in BMC2. The details of the data collection have been described previously. Baseline demographic, procedural, angiographic, and medication data are collected using a standardized data collection tool.
Medical records of all patients dying or who underwent coronary artery bypass grafting during the same hospitalization are reviewed by auditors from the co-ordinating center to review accuracy. Two percent of cases are also randomly selected for audit. The choice of the pharmacotherapy was at the discretion of the operating physician.
Inclusion criteria for this study included all patients who underwent PCI. Exclusion criteria were in-hospital death, documented contraindications to any of the 3 drugs, those who did not have any of the 3 drugs recorded, or who had >1 drug recorded.
The associations between subject demographics, clinical conditions, and the use of ticagrelor, prasugrel, and clopidogrel at discharge were examined using the chi-square statistic for categorical predictor variables and student t tests for continuous predictor variables. Current BMC2 random forest risk prediction models (available for review at https://bmc2.org/calculators ) were used to estimate the baseline patient predicted risks of postprocedural adverse events including contrast-induced nephropathy, transfusion, and death. The models used are the most recent iteration of ongoing work in this area by the BMC2 collaborative.
Results
From January 1, 2012, to March 31, 2014, there were a total of 68,983 hospital admissions in the BMC2-PCI database. Of these, the following groups were excluded: 1,093 patients who died in the hospital; 422 patients discharged alive who either documented contraindications to any of the 3 drugs; 2,669 patients who did not have any of the 3 drugs recorded as discharge medications; and 199 patients who had >1 drug recorded. The remaining 64,600 patients comprised our study population.
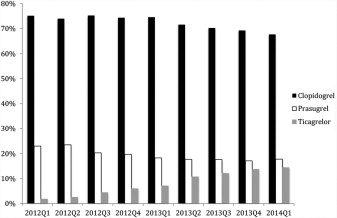
The proportion of patients receiving clopidogrel, prasugrel, and ticagrelor was 72.5% (n = 46,864), 19.5% (n = 12,596), and 8.0% (n = 5,140), respectively. The frequency of use of the 3 antiplatelet drugs by quarter is shown in Figure 1 ; quarterly use of ticagrelor increased from 1.8% to 14.4% during the study period.
Patient demographics and clinical characteristics are documented in Table 1 . Compared with patients receiving clopidogrel, those receiving ticagrelor were more likely to be younger and white. They also had less co-morbidity than patients receiving clopidogrel. Patients on ticagrelor had lower rates of hypertension, hyperlipidemia, previous myocardial infarction (MI), previous heart failure, previous PCI, previous coronary artery bypass grafting, cerebrovascular disease, peripheral arterial disease, chronic lung disease, and diabetes mellitus (p <0.001 for all). Patients receiving ticagrelor were more likely, however, to present with ST-segment elevation MI or equivalent or have cardiac arrest within 24 hours (p <0.001). Patients receiving clopidogrel more likely have had heart failure within 2 weeks of presentation and cardiomyopathy on admission.
| Patient Characteristics | Ticagrelor (n = 5,140) | Prasugrel (n = 12,596) | Clopidogrel (n = 46,864) | ||
|---|---|---|---|---|---|
| p ∗ | p ∗ | ||||
| Age (years) | 63.6 ± 12 | 59.4 ± 9.8 | <0.001 | 66.6 ± 12 | <0.001 |
| Sex (women) | 1,692 (32.9%) | 3,349 (26.6%) | <0.001 | 16,442 (35.1%) | 0.002 |
| White | 4,607 (89.6%) | 11,302 (89.7%) | 0.848 | 39,080 (83.4%) | <0.001 |
| African American | 382 (7.4%) | 963 (7.6%) | 0.626 | 6,192 (13.2%) | <0.001 |
| Current/Recent smoker | 1,466 (28.5 %) | 4,389 (34.8%) | <0.001 | 13,085 (27.9%) | 0.371 |
| Hypertension † | 4,190 (81.5 %) | 10,070 (80.0%) | 0.018 | 41,075 (87.7%) | <0.001 |
| Dyslipidemia ‡ | 3,962 (77.1%) | 9,942 (79.0%) | 0.006 | 39,345 (84.0%) | <0.001 |
| Pre-procedure creatinine | 1.06 +/- 0.78 | 1.03 ± 0.60 | 0.014 | 1.21 ± 1.08 | <0.001 |
| Family history of premature coronary artery disease | 898 (17.5%) | 2,736 (21.7%) | <0.001 | 8,346 (17.8%) | 0.553 |
| Diabetes mellitus | 1,688 (32.9%) | 4,467 (35.5%) | <0.001 | 18,741 (40.0%) | <0.001 |
| Prior myocardial infarction | 1,365 (26.6%) | 3,996 (31.7%) | <0.001 | 17,375 (37.1% | <0.001 |
| Prior heart failure | 509 (9.9%) | 1,155 (9.2%) | 0.127 | 8,807 (18.8%) | <0.001 |
| Prior percutaneous coronary intervention | 1,805 (35.1%) | 5,270 (41.8%) | <0.001 | 22,533 (48.1%) | <0.001 |
| Prior coronary artery bypass grafting | 692 (13.5%) | 1,628 (12.9%) | 0.338 | 9,770 (20.9%) | <0.001 |
| Cerebrovascular disease | 595 (11.6%) | 793 (6.3%) | <0.001 | 8,610 (18.4%) | <0.001 |
| Peripheral vascular disease | 505 (9.8%) | 1,265 (10.0%) | 0.664 | 8,697 (18.6%) | <0.001 |
| Chronic lung disease | 695 (13.5%) | 1,855 (14.7%) | 0.038 | 9,689 (20.7%) | <0.001 |
| Cardiomyopathy/left ventricular systolic dysfunction | 344 (6.7%) | 1,022 (8.1%) | 0.001 | 5,625 (12.0%) | <0.001 |
| Cardiogenic shock within 24 hours | 65 (1.3%) | 113 (0.9%) | 0.026 | 514 (1.1%) | 0.277 |
| Heart failure within 2 weeks | 362 (7.0%) | 717 (5.7%) | <0.001 | 5,932 (12.7%) | <0.001 |
| Cardiac arrest within 24 hours | 97 (1.9%) | 197 (1.6%) | 0.126 | 576 (1.2%) | <0.001 |
| Intra-aortic balloon pump | 85 (1.7%) | 175 (1.4%) | 0.185 | 816 (1.7%) | 0.647 |
| Percutaneous coronary intervention indication: ST- segment myocardial infarction | 1,170 (22.8%) | 2,142 (17.0%) | <0.001 | 5,505 (11.8%) | <0.001 |
| Percutaneous coronary intervention indication: rescue for ST-segment myocardial infarction (after failed full dose thrombolytics) | 35 (0.7%) | 77 (0.6%) | 0.394 | 206 (0.4 %) | 0.026 |
| Percutaneous coronary intervention indication: high risk non ST-segment myocardial infarction or unstable angina pectoris | 2,884 (56.1%) | 7,381 (58.6%) | 0.002 | 28,478 (60.8%) | <0.001 |
∗ The p-value reflects comparison to ticagrelor.
† Hypertension is defined as one meeting one of the following criteria: (1) History of hypertension diagnosed and treated with medication, diet and/or exercise; (2) Blood pressure greater than 140 mm Hg systolic or 90 mm Hg diastolic on at least 2 occasions; and (3) Currently on antihypertensive pharmacologic therapy.
‡ Total cholesterol greater than 200 mg/dL (5.18 mmol/l); or 2. Low-density lipoprotein (LDL) greater than or equal to 130 mg/dL (3.37 mmol/l); or 3. High-density lipoprotein (HDL) less than 40 mg/dL (1.04 mmol/l) or 4. Prior history of hyperlipidemia and currently on a statin. Data are expressed as proportion (%) or mean ± standard deviation.
Compared with patients on prasugrel, those on ticagrelor tended to be older, women, and white. Patients prescribed prasugrel were more often smokers, had a history of premature coronary artery disease, previous MI, and had previous PCI. Patients who received ticagrelor versus prasugrel had more acute presentations, including presenting with ST-segment elevation MI or equivalent, heart failure within the last 2 weeks, and cardiac arrest within 24 hours.
In our cohort, 53,564 procedures were performed for ACS. For all 3 presentations of ACS, clopidogrel was the most commonly used agent, whereas ticagrelor was least likely to be prescribed. Table 2 lists indications for receiving ticagrelor prasugrel and clopidogrel. Most notably, 13.6% of patients received ticagrelor for a non-ACS indication.



