Obesity is associated with increased asthma severity and worse asthma control. Nearly 60% of adults with severe asthma in the United States are obese [3] – these data were compiled when a little over 30% of the US population were obese [3], more recent data report 40% of the US population were obese [4], and so it is possible that the prevalence of obesity among patients with severe asthma is now even higher. Obese patients tend to have increased use of rescue medications, more exacerbations, and increased risk of emergency department visits and hospitalizations [5]. Among those hospitalized with asthma exacerbations, obese patients have a nearly twofold increased odds of requiring non-invasive mechanical ventilation and experience a longer hospital length of stay [6].
12.2 Physiology of Obese Asthma
The effects of obesity on airway physiology start in childhood: obesity in children is associated with accelerated lung growth, with FVC increasing more than FEV1. This differential airway growth – a phenomenon known as dysanapsis – can exaggerate airflow limitation and perhaps contribute to airway dysfunction [7, 8].
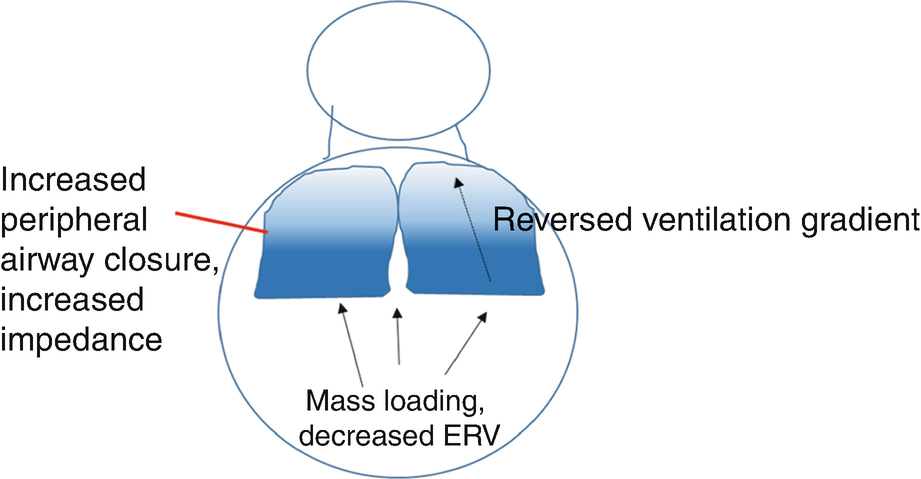
Physiologic changes in the lung in obese asthma: decreased lung volumes caused by mass loading, reversed ventilation gradient, increased peripheral airway closure, and increased impedance, particularly during bronchoconstriction
12.3 Mechanisms of Asthma in Obesity
Metabolic factors
Adipose tissue in obese individuals produces mediators known as “adipokines” and pro-inflammatory cytokines. These factors contribute to the metabolic complications of obesity such as diabetes mellitus, hepatic steatosis, and cardiovascular disease. These circulating factors likely also play a role in airway disease in obesity. For example, visceral fat inflammation is associated with airway reactivity [16], and circulating factors produced by adipose tissue are associated with asthma severity [17]. The exact mechanism by which these circulating metabolic factors affect the airway is an area of active investigation.
Immune abnormalities
Obesity skews away from prototypical allergic inflammatory pathways associated with asthma, enhancing Th1 inflammation [18], though overall effector T-lymphocyte function appears to be suppressed in obesity [19, 20]. Reports from animal models suggest augmented function of innate immune cells, such as ILC2 cells [21]. Markers of eosinophilic airway inflammation are typically decreased in obesity, although this may not correlate with overall systemic and mucosal markers of type 2 inflammation: airway trafficking of eosinophils may be altered – increased mucosal eosinophils, dissociated from sputum eosinophilia, but associated with sputum levels of interleukin 5, have been reported [22].
Increased oxidative stress
Obesity is characterized by increased levels of oxidative stress. Increased oxidative stress in the airway, associated with decreased levels of nitric oxide (an endogenous bronchodilator), likely also contributes to the pathogenesis of airway disease in obesity [23].
Diet
Obesity is associated with changes in dietary quality, which might significantly impact asthma. Obesogenic diet s are typically high in fat and low in fiber and anti-oxidants. Ingestion of a high-fat diet might impair bronchodilator responsiveness and increase airway inflammation [24]. Dietary factors also have long-term effects on the immune system through effects on the gut microbiome: studies in animal models suggest that high-fat and low-fiber diets both lead to changes in immune function which promote allergic airway inflammation [25, 26].
Genetic factors
Common genetic factors may predispose individuals to develop both obesity and asthma. In mice, genetic manipulation of the chitinase 3-like pathway increases susceptibility to both asthma and obesity, suggesting common genetic predisposition may be a factor in some individuals [27].
Environmental factors
Common exposures increase the risk of developing both obesity and asthma: parental smoking increases the risk of developing asthma (odds of having asthma increased 1.6-fold [28]), and maternal smoking confers a similar increased risk of childhood obesity [29]. Similarly, exposure to environmental air pollution confers increased risk of developing both asthma and obesity [30, 31]. Obese individuals also have exaggerated responses to air pollution, which contributes to morbidity in those with established disease [32–34].
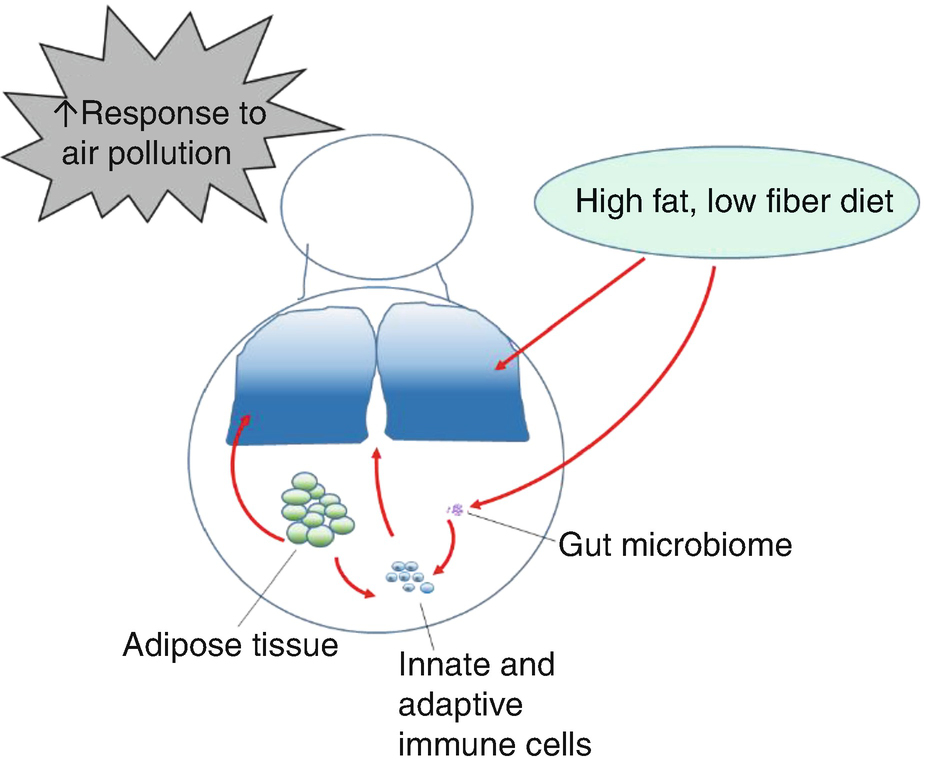
Factors contributing to pathogenesis of asthma in obesity: increased response to air pollution, acute and chronic effects of poor diet quality, long-term changes in the gut microbiome, changes in immune function, and factors released by adipose tissue all contribute to the pathogenesis of asthma in obese individuals
12.4 Drug Treatment
Response to corticosteroids
Inhaled corticosteroids and inhaled corticosteroid-long acting beta agonist combination therapy are not as effective in obese patients as in lean patients with asthma [35–37]: reports suggest lesser improvement in lung function, less of an effect on exhaled nitric oxide, and attenuated improvement in asthma symptoms [38]. Part of this may be related to different disease pathogenesis: there appear to be many patients with older onset of asthma, with minimal type 2 inflammation – the type which responds to inhaled corticosteroids (ICS). Another reason may be altered deposition of inhaled medications – in severe obesity, ventilation goes preferentially to the upper lung zones, in contrast to leaner patients in whom ventilation is greater in the lower lung zones [14, 39]. Indeed Anderson and Lipworth found a trend toward attenuated urinary cortisol suppressions in obesity which they thought might be related to reduced peripheral deposition and absorption of inhaled corticosteroids in heavier patients [40]. Another reason for attenuated response is intrinsic steroid resistance in obesity. There may be a number of factors that contribute to intrinsic corticosteroid resistance. One factor is impaired induction of anti-inflammatory pathways, as shown by studies of in vitro responses of alveolar and peripheral blood mononuclear cells to dexamethasone [41]. Another factor is altered pharmacokinetics: Goleva et al. reported that circulating corticosteroids were lower in direct proportion to BMI following oral administration of prednisone; this likely has therapeutic significance, as bronchoalveolar lavage TNFα was higher in patients with accelerated clearance [42]. While there are a number of studies suggesting that corticosteroids might be less effective in the obese, few have investigated whether increased dosing might be of use in this patient population – in a retrospective analysis, we found that quadrupling the dose of ICS seemed to have some efficacy in maintaining symptom control in obese patients (Dixon, personal observations).
Response to treatment in obese children
Studies in children suggest that overweight pre-school children have similar improvement in asthma control and severity in response to ICS as lean patients [43], but in older children, being obese is associated with a decreased response to bronchodilator [44].
Response to leukotriene modifiers
Response to the leukotriene receptor antagonist montelukast does not differ across BMI groups, though overall inhaled corticosteroids and ICS-LABA are more effective than montelukast in obese patients [35, 37].
Response to anti-cholinergics
Animal data suggest that the obese state leads to enhanced vagal tone [45, 46]; data in people with asthma suggest that response to the long-acting muscarinic antagonist tiotropium is similar across BMI groups [47].
Response to biologics
Some studies report that obese patients have worse symptoms and more frequent exacerbations than lean patients treated with omalizumab [48, 49], whereas others report no difference across BMI groups [50]. One barrier to use of omalizumab is weight-based dosing, so more severely obese patients may not be able to receive this mediation. As of yet, there do not appear to be publications reporting on the relative efficacy of other biologics for obese compared with lean asthmatics.
Response to beta agonists
Fingleton et al. reported that obese asthmatics had a lesser spirometric response to inhaled beta agonists [51]. Desai et al. reported that obese (non-asthmatic) subjects had greater improvement in lung impedance measured by forced oscillation, but similar improvements in spirometric lung function with albuterol, suggesting that obesity may be affecting peripheral airway tone and that studies quantifying bronchodilator response only by spirometry may be missing an effect in obese patients [52].
12.5 Vaccination and Susceptibility to Infections
Obese patients have increased risk of viral infections such as influenza. Beck and colleagues reported that obese patients who receive the influenza vaccine are at increased risk of developing influenza-type illnesses [53], related to defective immune responses to vaccine [54]. Obese patients hospitalized with a respiratory tract infection are at increased risk of having respiratory syncytial virus detected, suggesting that obesity might also be a risk factor for this infection [55]. While it is important to be aware that obese patients may be at increased risk of viral infections such as influenza, as of yet there are no specific recommendations for management of this risk.
12.6 Role of Co-morbidities
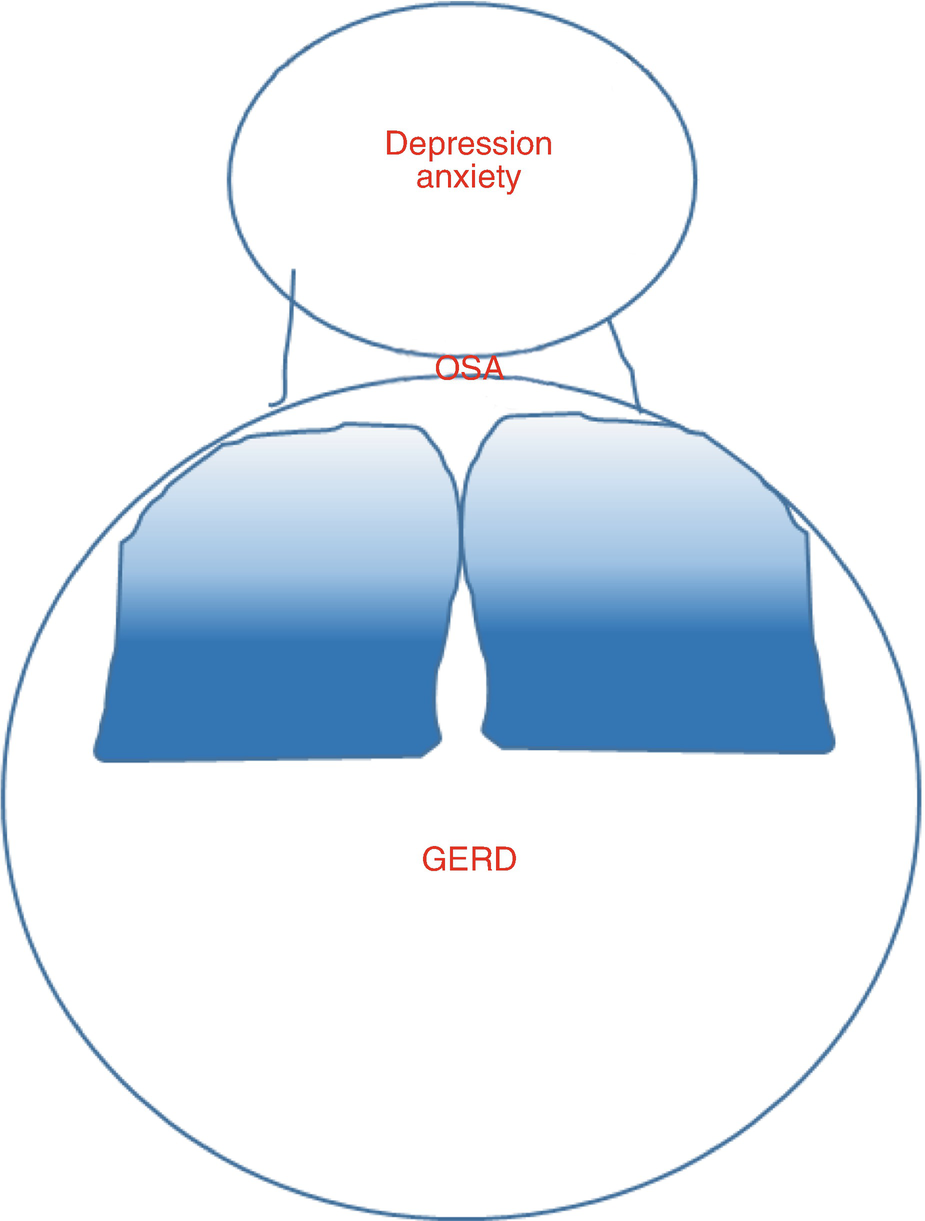
Co-morbidities increased in obese patients might lead to poor asthma control
Lang et al. have reported that symptoms of gastroesophageal reflux are particularly increased in obese children, and associated with asthma symptoms, but not lung function abnormalities [60]. In adults GERD is increased in obesity; however, esophageal probe measurement of GERD is not correlated with either asthma symptoms or lung function in patients without frequent GERD symptoms [61]. These data suggest that if obese patients have no overt symptoms of reflux, treatment of acid reflux is unlikely to affect asthma control, but in patients with severe reflux, it may be an important co-morbidity.
Both obesity and asthma are risk factors for the development of obstructive sleep apnea (OSA) [62]. Obstructive sleep apnea is associated with severe symptoms and airway neutrophilia, even independent of obesity status [63]. The presence of obesity combined with obstructive sleep apnea in patients hospitalized with asthma is associated with increased length of hospital stay and resource utilization [64]. Some small studies suggest that treatment of OSA might improve airway reactivity [65] and/or asthma quality of life in asthma [66]. As OSA should be treated regardless of asthma, there are unlikely to be many large, controlled studies examining the effects of treating OSA on asthma outcomes. As a practical approach, if a patient with asthma has obstructive sleep apnea, the OSA should be treated – it is reasonable to anticipate this might improve asthma control.
12.7 Lifestyle Interventions
Studies of lifestyle interventions suggest that loss of 5–10% of body weight is required to improve asthma control [67–69]. One study found that exercise added to the weight loss program might better improve asthma control [69]; this might relate to improved weight loss or perhaps immune effects of exercise. There are a number of studies in lean animal models suggesting exercise may reduce type 2 airway inflammation and reduce airway reactivity [70–72]. Similarly, when obese mice are exercised, airway reactivity, remodeling, and inflammation are reduced compared to obese sedentary mice [73]. In non-obese humans, exercise improves airway reactivity and inflammation [74]. Combined, these data suggest that weight loss through diet and perhaps particularly weight loss through diet with exercise are effective in improving asthma control.
Achieving weight loss can be challenging, so there has been recent interest in trying to understand whether improving dietary quality, rather than weight loss, might be effective. One pilot study randomizing patients with asthma to improved dietary quality compared with regular diet did find evidence of some efficacy for the treatment of asthma [75]. Similarly, a diet high in fruits and vegetables (but not simply supplementation with anti-oxidant) reduced asthma exacerbations [76]. These data suggest that dietary quality might affect long-term asthma outcomes such as symptoms and exacerbations.
There are also data suggesting that dietary factors can acutely affect airway inflammation and response to bronchodilator: Wood et al. compared the effects of a high-fat diet on airway inflammation and bronchodilator responsiveness in patients with asthma (not limited to obese patients) and found that the high-fat diet induced airway neutrophilia and impaired response to bronchodilator. The same group also compared the effects of a low- versus high-fiber challenge and found that a high-fiber challenge reduced airway inflammation [77]. These data suggest that diet can have short-term effects on airway inflammation and response to bronchodilators.
Lifestyle interventions are rarely considered in the management of patients with asthma and yet may be critical in this particular patient population. Physicians caring for obese patients with asthma should have a knowledge of local and/or online resources available to promote lifestyle change and consider partnering with health-care professionals with such expertise to successfully manage obese patients with asthma.
12.8 Bariatric Surgery
A number of studies have shown improved asthma control following bariatric surgery. We have found that improvement in airway responsiveness occurs particularly in those with later-onset asthma and low immunoglobulin E levels (a marker of atopy). In patients with poorly controlled asthma that cannot lose weight, particularly those with other co-morbidities, bariatric surgery may be a reasonable intervention. Studies suggest a significant decrease in asthma exacerbations after bariatric surgery [78], and numerous studies support favorable outcomes with numerous other co-morbidities [19, 79].
12.9 Conclusions
Obesity greatly complicates the management of asthma, with patients typically suffering from poor asthma control and increased severity. Some medications may not work as well in this patient population, likely related to intrinsic steroid resistance, altered pharmacodynamics, and also to altered pathophysiology of disease. Management of co-morbidities such as obstructive sleep apnea and depression may be particularly important in this patient population. Lifestyle modification is critical, and while weight loss is desirable, focusing on improving dietary quality may also be very important. Exercise is likely to be efficacious in the patient population. Weight loss, produced either through lifestyle interventions or bariatric surgery, should be considered as an important management strategy that will have benefits not only for the patient’s asthma but other co-morbidities and long-term health outcomes.

Full access? Get Clinical Tree


