Induction of TH2 cells . TSLP, IL-25, and IL-33 produced by respiratory epithelium on exposure to respiratory allergens, pollutants, or viruses promote the induction of allergen-specific TH2 cells. Activated allergen-specific TH2 cells produce IL-4, IL-5, IL-9, and IL-13 which act on respiratory epithelium, B cells, eosinophils, and mast cells to induce allergic airway inflammation
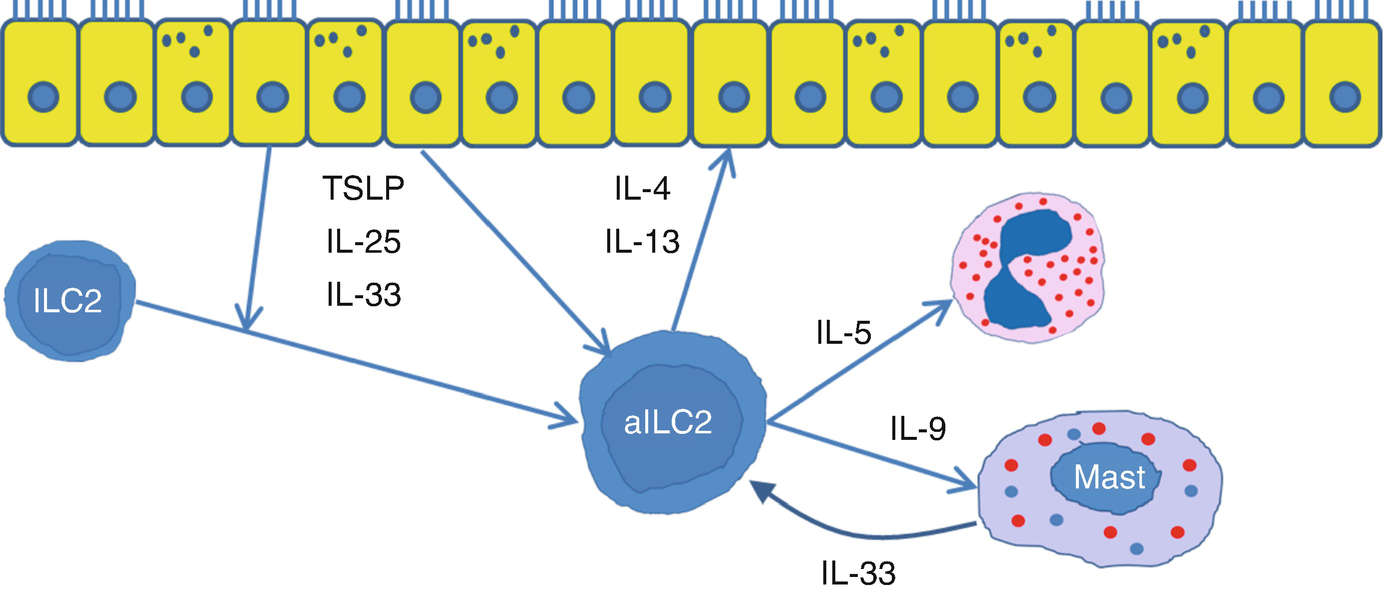
Induction of ILC2 cells . TSLP, IL-25, and IL-33 produced by respiratory epithelium on exposure to respiratory allergens, pollutants, or viruses promote the activation of ILC2 cells. Activated ILC2 cells produce IL-4, IL-5, IL-9, and IL-13 which act on respiratory epithelium, eosinophils, and mast cells to induce allergic airway inflammation
9.3 Diagnosis and Characterization of Allergic Asthma
The identification of allergic asthma in clinical practice should rest on two criteria: (1) documentation that symptoms are temporally induced with exposure to the sensitizing aeroallergens and (2) documentation of allergic sensitization with either immediate hypersensitivity skin tests or by in vitro testing for specific IgE in the serum.
9.3.1 Documentation that Symptoms Are Temporally Induced with Exposure to the Sensitizing Aeroallergens
Relevant questions to assess environmental exposures
1. Do you have indoor pets? If so, where? Do they go into the bedrooms? |
2. Do you get worse around your pets? Around other people’s pets? |
3. Have you seen cockroaches in your home? |
4. Have you seen rodents or their droppings in your home? |
5. Are your symptoms consistently worse in the early spring, last spring, or fall? |
6. Has there been any water problem or dampness in your home from ground water, rain, flooding, or leaks? |
7. Have you had problems with water damage? |
8. Have you noticed a moldy or musty smell inside your home? |
9. Have you noticed any large area of fungal growth (size of notebook)? |
10. Do you get better if you are away from home or work for a week or two? |
11. Do you have more symptoms when you are home? If so, where? |
12. Do you have increased symptoms at work? If so, what are you exposed to at work? |
9.3.2 Documentation of Allergic Sensitization
Testing for allergen-specific IgE is an essential part of the workup of patients with asthma. In vitro testing is a reasonable alternative to skin testing as it can be done in any clinical practice and patients do not have to stop antihistamines. It is also preferred in patients with severe airway disease when skin testing may be risky, as well as in those with extensive dermatitis. Although testing for specific IgE is usually relatively easy, determining the relationship between symptoms and exposure can be very challenging especially in those patients with perennial allergies or in the presence of multiple sensitivities.
- 1.
Pets – dog, cat
- 2.
Pests – mouse, rat, cockroach
- 3.
House dust mites – Dermatophagoides farina, Dermatophagoides pteronyssinus
- 4.
Pollens – mixed trees, grass mix, ragweed
- 5.
Molds – Alternaria, Cladosporium, Penicillium, Aspergillus
In addition to evaluation of allergen sensitivity and exposure, characterization of allergic asthma should include evaluation of severity (impairment and risk), current control, current and past medications and their effectiveness, results of pulmonary function tests, history of triggers, comorbidities, and patient/family understanding of asthma management. These aspects of evaluation are covered in other chapters.
9.4 Indoor Allergens and Allergic Asthma
When patients have acute asthma on exposure to an allergen that is readily identified in the environment, e.g., pets at a relative or friend’s house, the efficacy of avoidance is obvious. Similarly, in occupational asthma where acute attacks or persistent symptoms are closely tied to exposure at work and not elsewhere, complete avoidance is very feasible and has a major impact. Thus, there are situations where allergen-specific interventions are critically important and strongly recommended. However, systematic reviews of allergen-specific environmental interventions in asthma have generally provided weak or no evidence of benefit and recent GINA guidelines reflect this skepticism [9]. This is in contrast to the strong recommendations included in guidelines and reviews on the management of allergic occupational asthma. In many cases, occupational asthma is also allergic asthma and in some cases involves the same allergens as nonoccupational asthma. The lack of enthusiasm for environmental controls for nonoccupational allergic asthma is somewhat understandable given the challenges of study design and implementation, but there is sufficient evidence to say they can work and can be an important part of management.
Designing high-quality studies of allergen avoidance for allergic asthma in the home or other environments can be challenging. Blinding seriously limits the types of interventions that can be undertaken and sometimes randomization is difficult, e.g., there can be no blinding that the cat or dog has been removed from the home and randomization of pet removed would be enormously challenging. Thus, it is unlikely there will ever be a level A study of pet removal. Selecting the right population can also be especially daunting. To study a single intervention, you need a population that is sufficiently sensitized to the allergen being studied and sufficiently exposed where you are conducting the intervention but not elsewhere, not too sensitized and exposed to other aeroallergen, and not exposed to the study allergen for so long and/or at such a high concentration that removal no longer makes any difference. Moreover, the population needs to be sufficiently severe and not so aggressively treated that clinical events such as flare or Emergency Department/Urgent Care visits can be assessed with a reasonably sized population and within a reasonable time. With all these challenges it is remarkable that there are any high-quality studies that demonstrate effectiveness of single interventions. Nevertheless, despite all of these problems there are a few studies that meet all of these criteria.
As discussed before, the clinical importance of an aeroallergen may be clear from the patient’s history. However, when there is chronic exposure to perennial allergens in the home, there may be sensitization and chronic airway inflammation without acute symptoms. In addition, high levels of many indoor allergens may be present in the home without the patient being aware. As a result, the patient’s clinical history may be insufficient to determine which indoor allergens are clinically important. Objective data about sensitization and indoor allergen exposure would be ideal. Skin prick testing at an allergist’s office and in vitro assays for IgE sensitization are readily available, and both of these services are usually covered by insurers. IgE sensitization to indoor aeroallergens can be extremely helpful in determining which allergens to target. Moreover, studies have shown that non-specific airway hyperresponsiveness and levels of allergen-specific IgE are the main determinants of the asthmatic response to an inhaled allergen challenge [10, 11]. Furthermore, there are services that will measure indoor allergen levels in settled dust. However, except for a home assay for house dust mites, these services are research tools and not covered by insurers. Therefore, advice about levels of indoor aeroallergens is often based on a general impression about the patient’s home environment from history and knowledge about local conditions. A formal home visit as described by the EPA may help identify environmental exposures. Improvement of symptoms after environmental controls have been implemented should help confirm the clinical impression. However, environmental controls can be quite burdensome and are frequently, but not always, beyond the patient’s means.
Evidence for exposure to indoor allergens and asthma exacerbation (++ sufficient evidence, + suggestive, or limited evidence)
Sensitized | Nonsensitized | Nonantigen | |
|---|---|---|---|
Causal | |||
House dust mites | ++ | + | |
Cat | ++ | ||
Cockroach | ++ | + | |
Environmental tobacco smoke (ETS) | ++ | ||
Rodent—Lab | ++ | ||
Associated | |||
Dog | ++ | + | |
Indoor fungi | + | + | |
Dampness | + | ++ | |
Nitrogen dioxide (NO2) | ++ | ||
Rodent—Home | + | ||
Endotoxin | ++ | ||
9.4.1 Furry Pets
More than 50% of US households have a dog or a cat and approximately 12% of the population are sensitized. Of the asthma attacks, 44% were attributable to exposure to high levels of dog allergen in the bedroom in patients with asthma sensitive to dog, and 30% were attributable to cat allergen exposure among cat-sensitive patients. These results suggest in the US population more than one million increased asthma attacks each year for the dog-sensitive and exposed group and more than 500,000 increased asthma attacks for the cat-sensitive and exposed population of patients with asthma [13]. Of particular concern is the possibility that long-term exposure to furry pets in sensitized individuals may lead to accelerated decline in lung function. In a study of occupational exposure to laboratory animals, an excess decline of FEV1 (83 ml/yr., p < 0.05) and FVC (148 ml/yr., p < 0.01) was found in workers who were both sensitized and exposed.
The amount of pet allergen found in bedroom dust can be surprisingly high. The level of dog allergen (Can f1) was greater than 13 μg/gm of dust in 25% of US homes and greater than 112 μg/gm of dust in 10% of US homes. Similarly high levels were seen for cat allergen (Fel d 1), i.e., greater than 9 μg/gm of dust in 25% of US homes and greater than 50 μg/gm in 10% of US homes [14]. Complete removal of furry pets from the home can be effective in reducing levels of pet allergen. Serial house dust samples were collected from 15 homes before and after cat removal. Samples were obtained with a handheld vacuum cleaner, and allergen content was quantitated by a radioimmunoassay specific for the major cat allergen, Fel d 1. Baseline Fel d 1 content ranged from 7.8 to 437 FDA units per gram of dust with a median of 61.2 U/gm (1 FDA unit Fel d 1 = approximately 1 μg Fel d 1). Fel d 1 levels in 10 control homes without cats ranged from undetectable to 3.2 U/gm (median, 0.3 U/gm). After cats were removed Fel d 1 levels declined gradually over the next 6 months, with 8 of 15 reaching levels in control homes. Importantly allergen levels fell more rapidly and completely in houses with aggressive cleaning [15]. Removal of pets from the home can also improve symptoms and bronchial hyperresponsiveness, allowing reduction of maintenance therapy. In a controlled but very small and nonrandomized study, removal of furry pets from homes compared to keeping furry pets led to improvement in non-specific bronchial hyperactivity (p < 0.04). Moreover, all the subjects in the removal group were able to stop inhaled corticosteroids while all but one subject in the keeping group had to continue inhaled corticosteroids (p < 0.001) [16]. Thus, it is important to educate pet-sensitized patients and their families that removal of pets can dramatically reduce levels of pet allergen and that such intervention can contribute to asthma control even if the minority of patients follow such advice. Patients should also be educated about the risks of not removing pets. The worst-case scenario would be the analogy with occupational asthma. Failure to remove patients from exposure may mean that they lose the window of opportunity to reverse their airway reactivity and they are at risk for accelerated loss of lung function. If pets are not removed from the home of sensitized individuals, progression of disease needs to be closely monitored. Unfortunately, allergen reduction with pets still in the house is generally not satisfactory.
Interventions to reduce exposure to furry pet allergen
1. Remove furry pets from the home of pet-sensitized patients and have the house thoroughly cleaned including thorough vacuuming using HEPA filter, washing rugs and carpets, as well as dusting walls, blinds, and furniture. |
2. Cover air ducts in bedrooms with filters. |
3. Use allergy covers to encase any mattresses or pillows on which the pet has slept. |
4. Beware that basement or other areas where the pet has spent time are reservoirs that can spread allergen to the rest of the house. |
5. If the furry pet is kept in the house, keep it out of the patient’s room, cover mattress and pillows in the patient’s room with allergy covers, remove carpets and rugs from the patient’s room, and use a HEPA-filtered vacuum cleaner and a HEPA-filtered room air cleaner in the patient’s room. |
9.4.2 House Dust Mites
House dust mites have their own ecology and exposure to human populations varies widely throughout the world and over time. Humidity has a major effect, dry deserts and mountains generally having the lowest levels of house dust mites and humid coastal areas the highest. In Australia coastal cities have high humidity (Sydney average humidity 70%) and inland regions have low humidity (Broken Hill average humidity 47%). In a multicommunity study house dust mite levels and sensitization to house dust mites were much higher in the coastal cities. Bronchial airway hypersensitivity was directly related to the size of house dust mite skin prick tests (p < 0.001). The prevalence of current asthma was directly related to the level of house dust mites in dust (current asthma vs. levels of dust mite allergen r 2 = 0.82, p = 0.013) [17]. A particularly notable example of epidemic asthma comes from the study of asthma rates in certain villages in central New Guinea. After cloth bedding was introduced, rates of asthma in the adult population jumped from 0.1% to 7% over 20 years [18, 19]. Villages that had epidemic levels of asthma also had high levels of house dust mites in bedding and high levels of specific IgE to mites while control villages with low rates of asthma had markedly lower levels of house dust mites and mite sensitization. Interestingly, house dust mites also appear to be the dominant aeroallergen in another tropical but very advanced country, Singapore (average humidity 76%). Extraordinarily high rates of sensitization to house dust mites (70%) and very high levels of mite-specific IgE in sensitized individual have been found in the Chinese population of Singapore [20]. The next highest rates of sensitization were to German cockroach (15%) and Bermuda grass (7%) with specific IgE levels 8–30 fold lower. Thus, the population in Singapore could almost be considered mono-sensitized. Interestingly Chinese immigrants from the mainland compared to Singapore start with low levels of sensitization but after several years in Singapore develop the same high rates seen in natives. Thus, areas of high humidity have high levels of house dust mite, high rates of allergic sensitization to mites, and a correlation between mite sensitization and asthma.
Dust mite mitigation strategies
1. Cover mattresses, pillows, comforters, and box springs with fine woven fabric allergy covers. |
2. Wash bedding (sheets, pillow covers, blankets, mattress covers) in hot water every 2 days to 2 weeks. Heating blankets in a clothes drier for 10 minutes before washing is also effective. |
3. Keep relative humidity between 35% and 50%. |
4. Vacuum with HEPA filter. |
5. If possible remove carpets, large area rugs, upholstered furniture, clutter, etc.. |
9.4.3 Cockroach
Cockroach allergy is particularly important in inner city asthma. In a multicenter study, 37% of inner city asthmatic children were sensitized to cockroach and 50% had high bedroom levels of cockroach allergen [22]. Inner city asthmatic children who were both allergic to cockroach allergen and exposed to high levels of roach allergen had 0.37 hospitalization a year, as compared with 0.11 for the other children (p < 0.001), and 2.56 unscheduled medical visits for asthma per year, as compared with 1.43 (p < 0.001). These patients also had more days of wheezing, missed school days, and nights with loss of sleep. Also 35% of inner city asthmatics were also sensitized to house dust mites and 23% were sensitized to cat, only 10% and 13% of bedrooms had high levels of these allergens, and mite and cat allergen were not associated with worse outcomes. Thus, cockroach is associated with poor control of inner city asthma, a population with a relatively high rate of sensitization and exposure to cockroach compared to other common indoor allergens.
Integrated pest management for cockroach and rodents
1. Keep counters, sinks, tables, and floors clean and free of clutter. |
2. Clean dishes, crumbs, and spills right away. |
3. Store food in airtight containers. This also applies to pet food. |
4. Seal cracks or openings in cabinets, walls, baseboards, and around plumbing. |
5. Keep trash in a closed container. |
6. Use pesticide baits and traps in areas away from children and pets. Follow manufacturer’s instructions for correct use. |
What is fascinating about this New Orleans cockroach intervention study was that although benefits were greater in patients sensitized to cockroach, even patients not sensitive to cockroach had some benefit suggesting that cockroach can have a non-specific effect on asthma. Many cockroach allergens can activate many of the components of the innate immune system. In particular, Per a 10, a serine protease, has been shown to disrupt the respiratory epithelial barrier and induce secretion of TLSP and IL-33, two cytokines that promote TH2 differentiation [24]. Thus, cockroach allergen and many other aeroallergens can have direct effects on asthmatic airways in the absence of sensitization.
9.4.4 Rodents
Children sensitized and exposed to mouse allergen have increased asthma severity. Low-income (62%), minority (70% African Americans and 21% Hispanic) children and adolescents from inner city Baltimore and Boston with persistent asthma were recruited for evaluation of mouse sensitization and exposure [25]. Positive prick skin test to mouse was found in 54% and was associated with greater disease severity and higher level of treatment. Among mouse-sensitized individuals, higher levels of mouse allergen were also associated with increased disease severity and higher treatment level. There was also a statistically significant lower pre-bronchodilator FEV1/FVC with increasing age for mouse-sensitized (p < 0.001), but not nonsensitized participants (p < 0.26).
Reduction of mouse allergen levels are associated with reduced asthma symptoms, medication use, and acute care visits. In a randomized controlled study, professional integrated pest management (p-IPM) was compared to an educational program on environmental controls [26]. Children with persistent asthma and an asthma exacerbation were screened for sensitization to mouse allergen and for the presence of significant mouse allergen in their home. Subjects with both sensitization and exposure to mouse allergen were recruited into the study. The primary finding was p-IPM was no better than education in maximal symptoms days over 12 months. However, although there was also no difference in reduction of mouse allergen level between groups, both p-IPM and education resulted in significant decrease in mouse allergen from baseline (67% vs. 62% reduction of bedroom floor mouse allergen). Moreover, irrespective of group, reduction of mouse allergen level was associated with improvement in asthma control, e.g., a 90% reduction in mouse allergen resulted in a statistically significant reduction in maximal symptom days, use of rescue inhaler, exercise-related symptoms, and acute care visits [26]. Thus, for mouse infestation it appears that the educational program may be just as successful as professional integrated pest management.
9.4.5 Mold
Kanchongkittiphon’s review of environmental exposures on exacerbation of asthma concluded that, “There is sufficient evidence of a causal association between outdoor culturable fungal exposure and exacerbation in asthmatics sensitized to fungi,” but that the evidence for an association between indoor fungi and exacerbation of asthma was limited and only suggestive [12]. In contrast, a review of indoor “dampness or mold” was “associated consistently with increased asthma development and exacerbation, current and ever diagnosis of asthma, dyspnea, wheeze, cough, respiratory infections, bronchitis, allergic rhinitis, eczema, and upper respiratory tract symptoms,” and that this association was seen in both allergic and non-allergic individuals. Furthermore, evidence “strongly suggested causation of asthma exacerbation in children” [27].
Strategies to reduce mold exposure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


