7 Conotruncal Lesions
Tetralogy of Fallot
Background
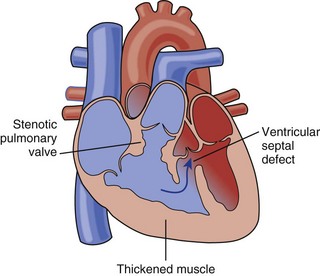
• The four historically described features of tetralogy of Fallot (TOF) are ventricular septal defect (VSD), overriding aorta (Ao), pulmonary stenosis (PS), and right ventricular hypertrophy (RVH) (Fig. 7-1).
• However, it is better for the echocardiographer to understand the key features:
• Large VSD of the anterior malalignment type (the conal or infundibular portion of the ventricular septum [VS] is anteriorly displaced).
• Underdevelopment of the right ventricular outflow (RVO), which can lead to obstruction at the subvalvar, valvar, and supravalvar levels.
• A continuum of severity exists.
• “Pink tets” have little in the way of PS, exclusively left-to-right shunting and physiology more akin to a large VSD.
• The typical patient with TOF has an intermediate degree of obstruction, which usually progresses with time.
• Toward the severe end of the spectrum, there may be critical degrees of obstruction, requiring ductal patency for adequate oxygenation.
• In the extreme, there is pulmonary atresia. There may be no identifiable central pulmonary arteries (PAs); the lungs are supplied by multiple collateral vessels, termed major aortopulmonary collaterals (MAPCAs). This anatomy is also termed pulmonary atresia/VSD, distinguishing it from pulmonary atresia with an intact VS.
• A notable variant is TOF with absent pulmonary valve (pulmV) syndrome.
• The pulmonary valve leaflets are vestigial. In utero, there is free pulmonary insufficiency (PI) and usually a relatively mild degree of stenosis.
• The branch PAs are often severely dilated and may compress the airways, leading to severe respiratory distress at birth.
• A typical TOF repair involves closing the VSD to provide unobstructed outflow from the left ventricle (LV) to the aorta and relief of all levels of pulmonary valve stenosis, often using a transannular patch.
Overview of Echocardiographic Approach
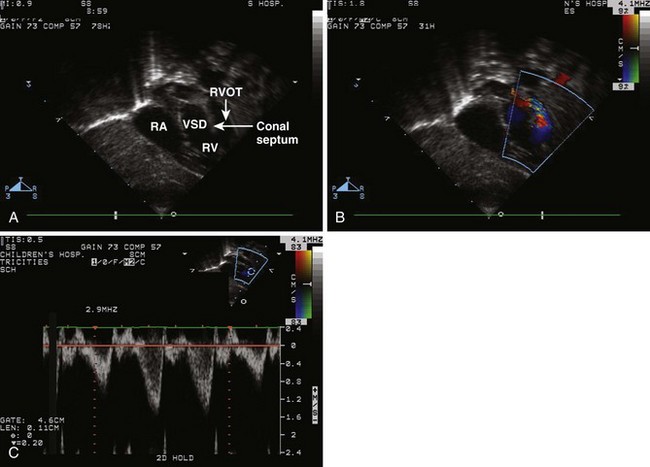
• Demonstrate the anterior malalignment of the conal septum and the various levels of RVOT obstruction (2D, color Doppler, pulsed wave [PW] Doppler, and continuous wave [CW] Doppler).
Anatomic Imaging
Acquisition
• Beginning with the subcostal views, evaluate the segmental anatomy.
• Perform careful 2D sweeps in the frontal (long axis) and bicaval (sagittal) planes, noting the morphology and relationship of structures. The segmental anatomy is normal (see Chapter 1, The Pediatric Transthoracic Echocardiogram).
• In addition, perform a true subcostal short axis (45 degrees between the frontal and bicaval planes) 2D sweep from base to apex. This can be particularly helpful in visualizing:
• Mitral-aortic fibrous continuity (if no continuity, consider the diagnosis of double-outlet right ventricle [DORV]).
• Parasternal short axis view (Fig. 7-4)
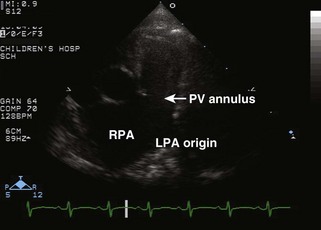
• Note any evidence of RVOTO, pulmonary valve hypoplasia/stenosis, supravalvar stenosis, and branch PA stenosis.
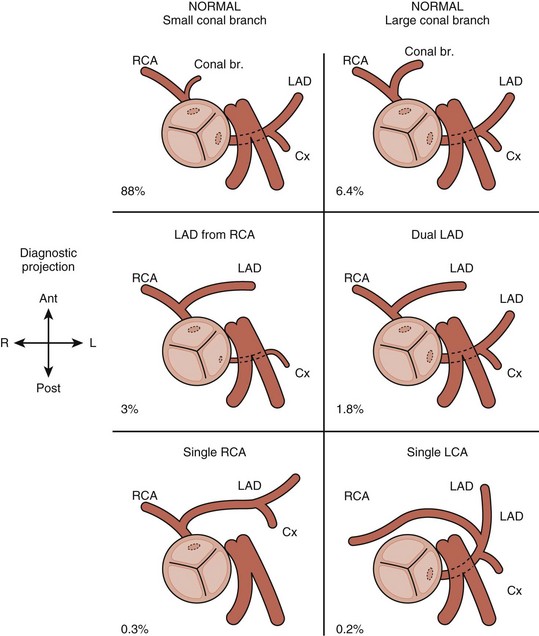
• Carefully image the coronary arteries (Figs. 7-7 and 7-8). In particular, demonstrate the origin and course of the anterior descending coronary and rule out any coronary artery crossing the pulmonary outflow tract. A significant coronary artery crossing the pulmonary outflow tract precludes a transannular patch. Decreasing the dynamic range and appropriate adjustment of focal zone and zoom features facilitates visualization.
• Short axis sweeps of the ventricles are used to evaluate function and any additional VSDs. Additional muscular VSDs may be difficult to detect by color Doppler imaging in the presence of another large VSD; thus, careful attention to the 2D images is mandatory.
• Suprasternal notch imaging
• Demonstrate arch sidedness and branching from the suprasternal short axis view. A right aortic arch occurs in about 25%. Some types of right aortic arch constitute a vascular ring. (See Chapter 1, The Pediatric Transthoracic Echocardiogram, for technique.)
Analysis
• Measure the malalignment VSD from the parasternal short axis 2D view and another orthogonal view (apical or subcostal).
• Measure the pulmonary valve annulus, main PA, and branch PAs from the parasternal views. Compare with norms based on body surface area (BSA) (Z scores).
Physiologic Data
• Subcostal views
• Perform color sweeps of the atrial septum (AS) and VS. Be alert for any additional muscular VSDs, which may be difficult to visualize because the ventricular pressures are typically equal due to the large primary VSD. Determine the direction and velocity of shunting across shunts. Generally, VSDs will be unrestrictive.
• Evaluate RVOT from the subcostal short axis view.
• This view provides good Doppler alignment for spectral analysis in the subvalvar and valvar region.
• Perform a careful PW analysis, starting within the right ventricle (RV) below any muscle bundles evident on 2D imaging, marching up through the RVOT and pulmonary valve. Use the 2D image to guide your placement of the Doppler cursor, thus demonstrating the significance of obstruction at the various levels.
• Apical views
• Assess tricuspid valve (TV) and mitral valve (MV) function with color Doppler. Note that obtaining a right ventricular (RV) pressure estimate is irrelevant in the presence of a large VSD. The RV pressure is equal to the left ventricular (LV) pressure.
• Parasternal views
• Assess outflow tract obstruction with color Doppler and spectral Doppler. In particular, pulmonary outflow obstruction may be assessed from the parasternal short axis. Use careful PW Doppler to localize various levels of obstruction. Use CW Doppler to assess highest velocity.
• Use color Doppler with very low Nyquist limit to assess flow in coronary arteries. Confirm that direction of flow is consistent with your understanding of the 2D anatomy. If not, reevaluate 2D anatomy.
• Suprasternal sagittal views
• Evaluate for PDA by color Doppler. If a ductus is present, demonstrate velocity and direction of shunting by spectral Doppler.
Alternate Approaches
• Cardiac catheterization with angiography is used:
• To definitively determine coronary artery anatomy if there is concern about an abnormal pattern or if the coronary arteries were inadequately imaged.
Key Points
• Key anatomic features
• Underdevelopment of the pulmonary outflow, which can lead to obstruction at the subvalvar, valvar, and supravalvar levels.
• Key aspects of the preoperative echocardiogram
• Evaluate for any coronary artery crossing the RVOT (present in ~5% of cases) that would preclude a transannular patch repair.
TOF Repaired
Background
• Repair of TOF entails the following:
• Establishment of unobstructed outflow from the RV to the PAs, tailored to the anatomy of the individual patient.
• In the best case, the pulmonary valve annulus is of adequate size and RV muscle bundles can be resected through the TV and pulmonary valve, avoiding right ventriculotomy (transatrial/transpulmonary repair).
• More frequently, the pulmonary valve annulus is hypoplastic, and a transannular patch is required. The surgeon incises from the main PA, across the pulmonary valve and onto the RV and then augments the area with a patch. Free PI results.
• If a coronary artery crosses the RVOT or if there is pulmonary atresia with no continuity of the main PA to the RV, then an RV-to-PA conduit is placed.
• The most important long-term follow-up issue in TOF is the development of RV dilation and dysfunction due to chronic PI and previous ventriculotomy. Serial evaluation of RV size and function is key.
Overview of Echocardiographic Approach
• Review the history if possible before beginning the study to understand what type of repair was performed.
• Evaluate RV size and function (2D). Estimate RV pressure if possible from the TR velocity (color Doppler, PW Doppler, and CW Doppler).
Anatomic Imaging
Acquisition
• Apical views
• Assess the size and function of both ventricles, in particular the RV.
• TOF repair often leads to long-standing severe PI, which can lead to right heart dilation and RV dysfunction.
• As the right heart dilates, the angle of the TV annulus may tilt, the RV develops a “shoulder” near the free wall TV annulus, and the apex becomes broader (Fig. 7-9).
• Parasternal views
• Optimize long axis 2D image for measurement of aortic annulus, root, sinotubular junction, and ascending aorta.
• The VSD patch can be visualized from long axis and short axis views; assess for any obvious residual VSD.
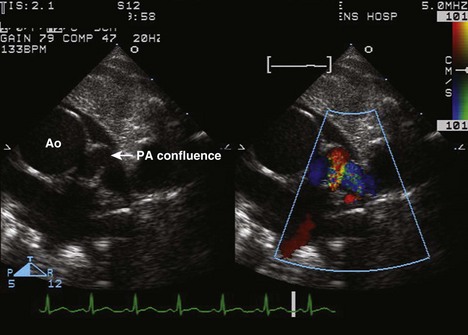
• Visualize the RVOT and branch PAs. Optimize view of branch PAs for measurement. If a conduit is present, pay attention to the motion of the conduit valve leaflets, which can become calcified and immobile over time.
• Assess the size and function of both ventricles.
• The RVOT in particular may demonstrate hypokinesis due to a history of ventriculotomy and the presence of a transannular patch.
• Suprasternal notch views: may be used to visualize branch PAs if not well seen from the parasternal short axis view.
Analysis
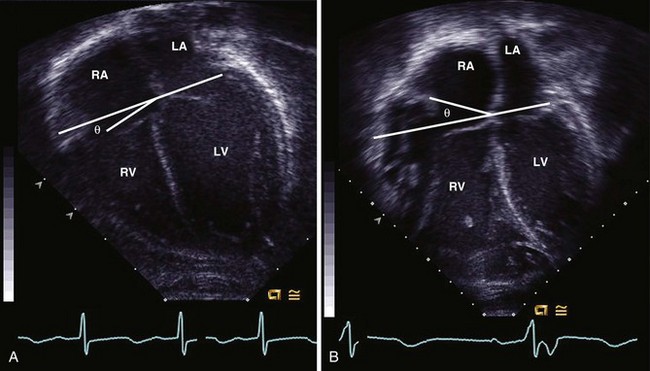
• In the apical view, assess TV annulus angle. An angle greater than 20 degrees has been associated with RV volume greater than 150 mL/m2 (Fig. 7-11).
• Measure LV dimensions in the parasternal short axis or long axis view. Calculate shortening fraction if septal wall motion is not paradoxical.
• Measure branch PAs from the parasternal short axis view or the suprasternal notch view. Compare with norms for BSA (Z score) in smaller patients. Usual norms can be used for average adult-sized patients.
Physiologic Data