Congestive Hert Failure Secondary to Congenital Heart Disease
Yuk M. Law
David J. Sahn
The management of patients with congestive heart failure (CHF) secondary to congenital heart disease must be examined at three interrelated levels: age, pathophysiological basis, and impact of surgery. For ease of reference and simplicity, this chapter will be organized by age. Although one pathophysiological state or surgery may predominate at one age, this interrelationship and its effects on medical management is a continuum through the different age groups.
As opposed to adults with heart failure from cardiomyopathy with systolic dysfunction, sufficient clinical trials in children with heart failure from cardiomyopathy or patients with congenital heart disease have not been done. However, because the success of modern treatment is pathophysiology-based, these different groups may still share certain common mechanistic pathways. Hence, it is important to understand the pathophysiological basis of their circulatory compromise so the proper extrapolation of data can be made in the management of the congenital patient.
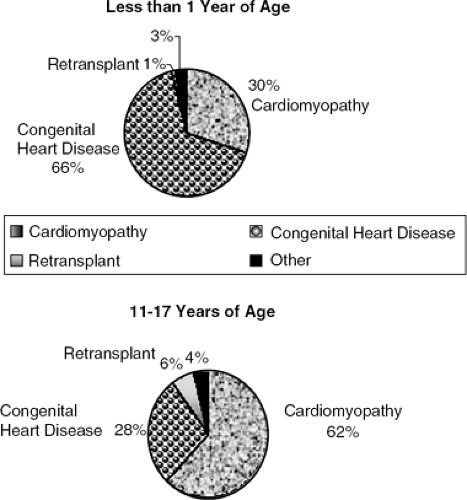
Table 20-1 lists the pathophysiological categories of heart failure seen in congenital heart disease. Unlike many of the disorders in adults with acquired heart disease (for which the development of major symptoms of CHF is necessary to warrant the risk of surgery), most congenital heart defects are corrected or palliated before the development of significant symptoms. Furthermore, since many patients can only be palliated, the strategic planning of interventions is critical, making the accurate diagnosis, monitoring, and coordination of medical, percutaneous, and surgical treatment paramount. When CHF supervenes late in the course of the disease, the opportunity for intervention (short of transplantation) may be primarily medical and supportive. Indeed, the indication for heart transplantation in children under one year of age is congenital heart disease, most of these patients deemed irreparable. The opposite is true for adolescents, and young adults, where cardiomyopathy is the main indication (Fig. 20-1).
Fetus
Fetal CHF is recognized as hydrops by the obstetrician or ultrasonographer. The fetus develops massive fluid accumulation in serous cavities and soft tissue and rapidly increases in size by examination and is large for dates. Ultrasound identifies the fetus as the source of the abnormal growth. Currently, at least 20% of the etiologies are cardiovascular (1) (Table 20-2). Because the mortality ranges from 50% to 98% in hydrops fetalis, early identification can allow pregnancy termination. Conversely, otherwise-healthy fetuses with readily correctable problems might be treated, for example, by surgical correction of urinary tract obstruction or chemical cardioversion of tachyarrhythmias. The possibility of fetal cardiac corrective surgery continues to be explored; however, these procedures are not yet ready for human trials (2,3). Percutaneous catheter balloon dilatation to promote growth of a strangulated ventricle and alleviate heart failure in critical pulmonic and aortic stenosis has not been successful (2). More recent interest is being placed on averting the lethal outcome before surgery in neonates with hypoplastic left heart syndrome and a highly restrictive patent foramen ovale by percutaneous balloon dilatation of the fetal foramen. In one study, the outcome was not improved in seven fetuses (4).
Fetal tachyarrhythmias, which can be easily diagnosed by fetal echocardiography (Fig. 20-2), have been the most
amenable to treatment in utero. The variety of arrhythmias encountered is shown in Table 20-3 (5). The vulnerability of the fetus to tachyarrhythmias has a physiological basis (6): as the fetal myocardium matures, calcium release and uptake shifts from sarcolemma to sarcoplasmic reticulum with the development of that organelle. Thus, the immature myocardium may be less able to relax at elevated heart rates due to the lack of development of the sarcoplasmic reticulum. Fetal muscle is also stiffer than neonatal or adult heart muscle and exhibits a reversed E wave to A wave (E/A) ratio on inflow Doppler imaging. This may result from the relative lack of fibril in the immature myocyte. Thus, fetal tachycardia can diminish ventricular filling, even at elevated pressures.
amenable to treatment in utero. The variety of arrhythmias encountered is shown in Table 20-3 (5). The vulnerability of the fetus to tachyarrhythmias has a physiological basis (6): as the fetal myocardium matures, calcium release and uptake shifts from sarcolemma to sarcoplasmic reticulum with the development of that organelle. Thus, the immature myocardium may be less able to relax at elevated heart rates due to the lack of development of the sarcoplasmic reticulum. Fetal muscle is also stiffer than neonatal or adult heart muscle and exhibits a reversed E wave to A wave (E/A) ratio on inflow Doppler imaging. This may result from the relative lack of fibril in the immature myocyte. Thus, fetal tachycardia can diminish ventricular filling, even at elevated pressures.
Table 20-1 Heart Failure in Congenital Heart Disease Based on Pathophysiological States | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 20-2 Causes of Nonimmune Hydrops | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Transplacental antiarrhythmic therapy is frequently employed to convert supraventricular tachycardia or to control the rate in atrial flutter or fibrillation. In Kleinman et al.’s series of 16 patients with atrial tachyarrhythmias, 15 were controlled with digoxin, verapamil, propranolol, procainamide, or their combination (5). The decision to treat is based on evidence of fetal distress (hydrops) and knowledge that premature delivery may have considerable complications. Thus, delivery is recommended only for failure of in utero rhythm management.
Neonate and Infant
The transition from intrauterine life to a terrestrial existence is accompanied by a cardiovascular revolution (6). Oxygen consumption is doubled because of the need for movement, respiration, digestion, and thermal regulation. Increase in the left ventricular (by 200%) and right ventricular (50%) output, as well as oxygenation of arterial blood, enables this demand to be met. With the onset of neonatal respiration, pulmonary vascular resistance falls and pulmonary venous return raises left atrial pressure, closing the foramen. Ligation of the cord eliminates the placental and venosus shunts. The patent ductus arteriosus (PDA) closes in response to elevated oxygen levels by 48 hours, and the fetal circulation has acquired the characteristics it will retain for the rest of life. However, if anatomic abnormalities exist, the circulation may not be able to adapt and heart failure may ensue. The early infancy period has by far the greatest incidence of CHF in the pediatric population (7). The common causes of heart failure during this period are listed in Table 20-4.
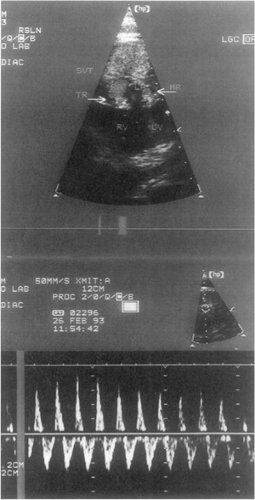 Figure 20-2 Fetal supraventricular tachycardia (SVT) with mitral regurgitation (MR) and tricuspid regurgitation (TR), precursors of hydrops. |
The persistence of the fetal circulation pathway in the premature or term infant (right-to-left shunting at the foramen ovale or PDA) is usually associated with important pulmonary disease such as congenital diaphragmatic hernia, sepsis, pneumonia, or meconium aspiration, resulting in persistent pulmonary hypertension of the newborn (8). These infants are hemodynamically compromised with combined cardiopulmonary failure and require pressor, inotropic, or ventilatory support, sometimes culminating in extracorporeal membranous oxygenation circulatory assist. In fact, this is the only indication for which inhaled nitric oxide is approved (9). Pulmonary disease can prevent the normal fall in pulmonary vascular resistance with birth, but other pulmonary diseases, such as premature lungs, are exquisitely sensitive to overperfusion through the PDA and heart failure is commonly seen, even with small shunts. Ductal closure using prostaglandin inhibition or surgery is then required.
Table 20-3 Fetal Arrhythmias | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Severe obstruction to flow through the left heart and aorta becomes manifest as heart failure shortly after birth because of the separation of the parallel circulation of the fetus into series circulation with the closure of the fetal shunts. In hypoplastic left heart syndrome, severe mitral stenosis, critical aortic stenosis, or coarctation of the aorta (Fig. 20-3), the right ventricle (RV) must supply blood to the body using the PDA in utero and postnatally (Fig. 20-4). These infants are critically ill and need prompt diagnosis and, if feasible, surgical palliation. Some of these infants are not recognized to have heart disease and are discharged from the nursery only to present in cardiogenic shock as outpatients when the ductus closes. This is one lesion whereby prenatal diagnosis by fetal echocardiography has utmost utility.
Table 20-4 Common Causes of Congestive Heart Failure in Infants with Congenital Heart Disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
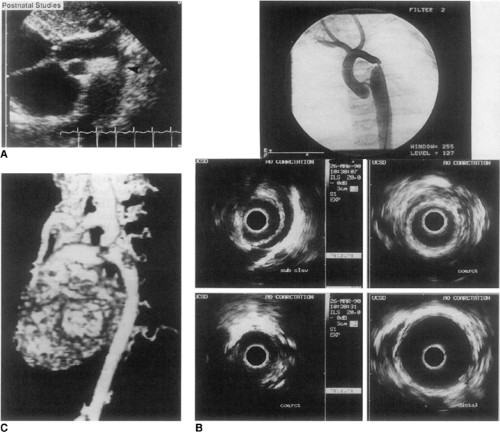 Figure 20-3 Severe coarctation imaged by ultrasound (A), intravascular echography and angiography (B), and three-dimensional reconstruction of magnetic resonance flow data (C). |
The progressive decline in pulmonary vascular resistance following birth invites increasing pulmonary blood flow when unrestricted communication exists between the high-pressure left ventricle (LV) or aorta and the RV or pulmonary artery. Although the systemic ventricle normally doubles its output at birth, pulmonary blood flow may exceed systemic blood flow by fourfold with large defects. In this setting, the systemic ventricle will be overloaded with pulmonary venous return and CHF ensues. These infants may appear well at birth when the pulmonary vascular resistance is still relatively high, and the defect may go unnoticed. In addition, the programmed fall in pulmonary vascular resistance can be retarded by increased flow and pressures, delaying the threshold of clinical presentation until weeks later. The lesions most commonly responsible for this form of high-output heart failure are large defects of the ventricular septum (VSD) or atrioventricular septum (ASD) (Fig. 20-5). Dextra-transposition of the great arteries (D-TGA) with a large VSD and truncus arteriosus may also produce heart failure with progressive fall in pulmonary vascular resistance, but cyanosis is usually the presenting sign. Total anomalous pulmonary venous return with obstruction can also present with cyanosis but florid pulmonary edema will predominate. It should be noted that ASDs, regardless of size, rarely result in heart failure at this age. Shunting at the atrial level is dependent on diastolic rather than systolic or arterial pressure differences. Left-to-right shunting occurs only to the extent that the RV is larger and more compliant than the LV. Therefore, overt heart failure in an infant with an ASD requires careful inspection for additional left-sided lesions (10). In addition, RV diastolic pressure cannot exceed LV diastolic pressure; thus, RV failure is rare unless severe pulmonary hypertension is present or until late in the natural history of this lesion.
Paroxysmal supraventricular tachycardia (SVT) may also cause CHF in infants. Whether the mechanism is dual atrioventricular (AV) nodal pathways or Wolf-Parkinson-White syndrome, the rapid ventricular response may be
poorly tolerated. Attention should be directed toward conversion to and maintenance of sinus rhythm, and delineation of any underlying cardiac abnormalities by echocardiography. Ebstein anomaly and cardiac tumors are known to be associated with SVT.
poorly tolerated. Attention should be directed toward conversion to and maintenance of sinus rhythm, and delineation of any underlying cardiac abnormalities by echocardiography. Ebstein anomaly and cardiac tumors are known to be associated with SVT.
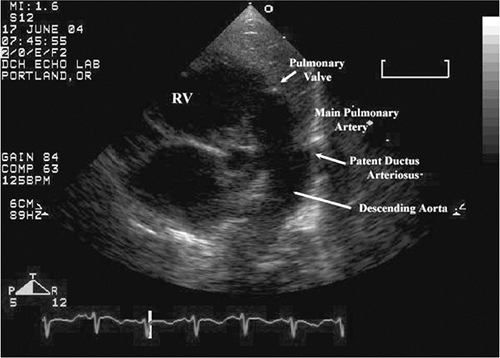 Figure 20-4 Right ventricle acts as “left ventricular assist” through the patent ductus arteriosus in hypoplastic left heart syndrome, a ductal-dependent lesion causing heart failure in neonates. |
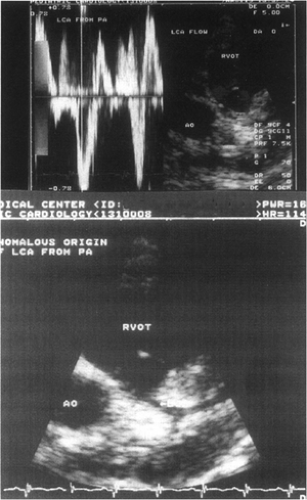
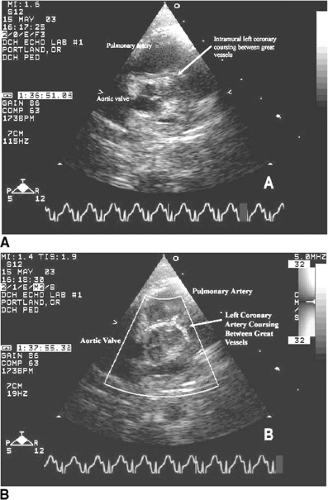
Other less-common causes of heart failure include congenital anomalies of the coronary arteries, mitral stenosis (Fig. 20-6), and supramitral stenosis from cor triatriatum. Anomalous origin of the left main or left anterior descending coronary arteries from the pulmonary artery (Fig. 20-7), or as a single right coronary artery (Fig. 20-8), usually presents with ischemia or infarction. Primary heart muscle and inflammatory diseases finish the list of differential diagnoses.
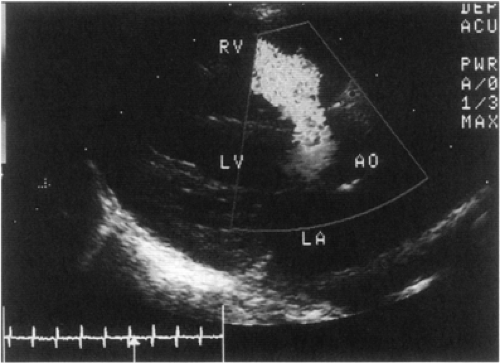 Figure 20-5 Moderate sized perimembranous ventricular septal defect (VSD) with color Doppler indicating a left-to-right shunt. |
Congenital cardiomyopathies can present with insidious or acute heart failure. Although these are too lengthy to describe here in detail, it is especially important in this age group that the diagnostic workup must consider cytogenetic aberrations, syndromes, neuromuscular diseases, and abnormal metabolic/endocrine pathways. Neonatal enterovirus infection can be devastating and can cause myocarditis. Maternal lupus antibody-mediated congenital complete heart block (11) is a unique pediatric entity that can also cause heart failure, even with prompt pacemaker implantation. Evidence points to prenatal maternal anti-SSA/Ro and anti-SSB/La antibodies attacking only fetal cardiomyocytes, leading to myocarditis or cardiomyopathy, as the disease does not exist in adults with lupus (12).
Diagnosis of heart failure in infants relies on echocardiography but it is nevertheless important to recognize the clinical manifestations (Table 20-5). Because young infants do not “walk up stairs,” a simple-to-use heart failure severity scale is difficult to develop; attempts have been made but none has been widely adopted (13,14,15). This further impedes the ability to systematically study the progression and success of various medical interventions. Of promise are heart failure biomarkers, such as B-type natriuretic peptide (BNP), used as a surrogate classification scale (16,17,18,19), but the proper accuracy studies must still be performed. Since the major functional activities in infants are feeding and growing, growth may be another accepted endpoint in future heart failure studies in this population.
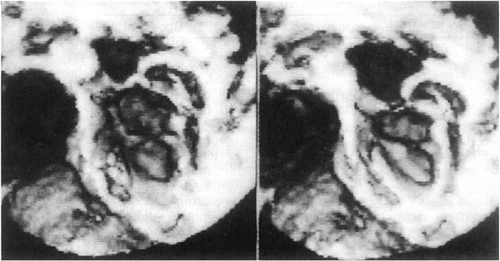 Figure 20-6 Three-dimensional echocardiographic reconstruction of severe congenital mitral stenosis in an infant. |
Table 20-5 Recognition of Congestive Heart Failure in Infants | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The management of infants with CHF should not be delayed; CHF is an emergency if there is hemodynamic compromise. Attention must be quickly directed to stabilization of respiratory, acid-base, and hemodynamic variables, followed immediately by anatomic definition of the underlying cardiovascular abnormalities by echocardiography. Surgical therapy is urgent, when appropriate. With improvement in echocardiography, catheterization and angiography are seldom needed. The general approach in this age group is not significantly different from that in other age groups. The major exceptions are (a) manipulation of the PDA; (b) cautious use of vasodilators; (c) insufficient evidence-based trial data; and (d) planning of intervention with surgery in mind. Relaxation of ductal constriction by prostaglandin E1 in hypoplastic left heart syndrome allows the right ventricle to continue its fetal role of supplying systemic blood flow, thus improving systemic perfusion. With severe juxtaductal coarctation, ductal relaxation (even if the ductus cannot be made patent) can sometimes allow enough antegrade flow across the coarctation. Conversely, premature infants with respiratory failure from left heart failure may benefit from ductal constriction with prostaglandin inhibition with indomethacin.
Vasodilator therapy for afterload or preload reduction is effective therapy for cardiac dysfunction caused by cardiomyopathy, ischemia, postcardiotomy, or severe aortic or mitral valve regurgitation (20). However, the high prevalence of heart failure caused by shunts and multilevel lesions in infants requires a secure anatomic diagnosis before empiric therapy is begun. Theoretically, systemic afterload reduction should help left-to-right shunt heart failure. However, properly controlled studies to assess its benefits beyond the acute setting are lacking.
Similarly, the proven benefits of beta-blocker therapy in adults with heart failure may also benefit selected infants with chronic heart failure. Studies have shown elevated catecholamine, inflammatory cytokine, and renin-angiotensin-aldosterone system (RAAS) levels in some congenital lesions (21,22,23,24). One controlled study was able to demonstrate the salutary effect of neurohormone inhibition in infants with shunt-related heart failure (25,26). Akin to the adult patient with systolic dysfunction, digoxin has been incorporated into the pediatric practice and theory of heart failure management, but its effectiveness has never been convincingly demonstrated. Under careful monitoring, patients who remain symptomatic after optimization may receive additional benefits from it. Nevertheless, diuretics to decongest pulmonary symptoms remain the cornerstone of pharmacologic therapy. Refer to The Harriet Lane Handbook (27) for pediatric dosing of standard cardiovascular drugs. Inotropes are rarely needed for high-output heart failure except in postoperative management or as rescue from cardiogenic shock. Milrinone has finally been shown to be advantageous in postcardiac surgery support of infants (28).
Child and Adolescent
The initial occurrence of CHF in a child with congenital heart disease without prior corrective or palliative surgery is uncommon. Therefore, in the diagnostic workup of CHF in this age group, acquired heart disease must be strongly considered. A description of acquired causes is beyond the scope of this book; nevertheless, several congenital defects may present with CHF in childhood or adolescence. As congenital heart repairs are now done earlier in life, infants who were repaired or palliated will also present in this age group with CHF secondary to the progression of residual abnormalities or to late postoperative complications.
Unoperated Patients
It must be remembered that this is a widely diverse group in terms of congenital heart disease (Table 20-6). Some individuals did not undergo an operation because their lesion was subclinical at an earlier age. Others simply are not amenable or would not be better off with any palliation. Also, some patients never had access to care, although this is less-often encountered in Western countries. The last two groups largely contribute to those who go on to develop Eisenmenger syndrome.
Pulmonary Hypertension and Eisenmenger Syndrome
Progressive pulmonary vascular obstructive disease occurs with high pressure and flow in the pulmonary circuit. This results from left-to-right shunting because of ventricular or great vessel communication. Although associated, atrial shunting alone is not usually the sole cause of severe pulmonary hypertension. With severely elevated and fixed pulmonary vascular resistance, pulmonary artery pressures equal systemic pressures and right-to-left shunting occurs, culminating with significant cyanosis and Eisenmenger syndrome. Pulmonic and tricuspid insufficiency is usually seen. In general, patients with Eisenmenger syndrome remain free of CHF much longer than those with idiopathic pulmonary hypertension, probably because the disease process is different and the former have biventricular assist
and pop-off across their defects. Therefore, intervention (short of heart-lung transplantation) is uncommon. However, children who have high resistance but reversible right-to-left shunting may still be able to have their defect closed if they demonstrate reasonable pulmonary vasoreactivity. The initial episode of heart failure may be heralded by atrial flutter, fibrillation, or ectopic tachycardia.
and pop-off across their defects. Therefore, intervention (short of heart-lung transplantation) is uncommon. However, children who have high resistance but reversible right-to-left shunting may still be able to have their defect closed if they demonstrate reasonable pulmonary vasoreactivity. The initial episode of heart failure may be heralded by atrial flutter, fibrillation, or ectopic tachycardia.
Table 20-6 Causes of Congestive Heart Failure in Children and Adolescents (Unoperated) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree