Congestive Heart Failure as a Consequence of Valvular Heart Disease
William J. McKenna
Denis Pellerin
Niall G. Mahon
Barry H. Greenberg
The management of patients with congestive heart failure (CHF) has become an international health care problem in the ever-aging population. In the United States, nearly 5 million people suffer from heart failure (HF) and fewer than 3,000 are offered transplantation due to limitations of age and the persistent and worsening shortage of donors.
Although the current epidemic of HF in developed countries is chiefly attributable to coronary disease and hypertension, valvular heart disease remains responsible for a significant proportion of cases (1). Valvular heart disease is a mechanical problem and, ideally, it warrants a mechanical solution. The art and science of managing valvular disease consist of determining the optimal time for surgery. In patients with valvular disease, the consequences of volume or pressure overload on left ventricular (LV) cavity size and function are the main prognosis indicators. The development of HF is usually the result of a severe impairment of valvular function that overwhelms the physiologic compensatory mechanisms that are activated for response to the increased load on the heart. Once HF has occurred, valvular heart disease is associated with a dramatic worsening of prognosis. Thus, prevention, prompt recognition, and appropriate treatment of HF in patients with valvular heart disease are of the utmost importance.
In this chapter, we will review specific issues related to chronic and acute HF as a consequence of valvular lesions of the left side of the heart. General principles and major issues of management are discussed. For further details, the reader is referred to the guidelines for the management of patients with valvular heart disease of the American College of Cardiology/American Heart Association (ACC/AHA) Task Force (2). Congestive HF secondary to valvular disease of the right side of the heart is discussed in another chapter in this text.
Cellular and Molecular Consequences of Chronic Valvular Disease
Volume overload stretches the myocardium and induces LV dilatation. Pressure overload increases wall stress and wall thickness. Cellular and molecular consequences of chronic valvular disease, including physiological response to pressure or/and volume overload, and progression from hypertrophy with small cavity to dilatation and dysfunction are not fully understood.
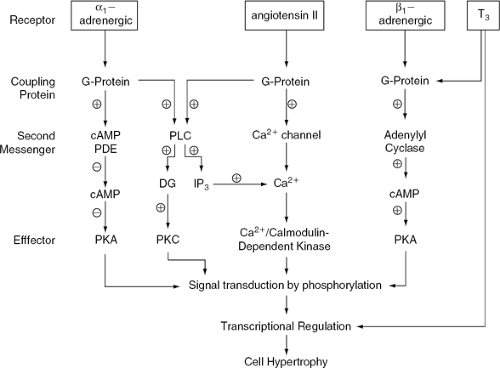
Because the cells of adult heart muscle are terminally differentiated and have lost their capacity to divide, wall thickness is increased through individual myocyte hypertrophy. This is a dynamic process resulting from an imbalance between protein synthesis and degradation, in which an increased number of sarcomeres provides a short-term adaptive response to overload. In this process, fetal muscle-specific gene products may reappear but, as the cell cycle remains blocked, cell division is not achieved (3). In chronic valve disease, pressure overload leads to concentric hypertrophy and volume overload leads to dilatation and eccentric hypertrophy. A notable difference between these two patterns is the spatial arrangement of the sarcomeres; in concentric hypertrophy they are arranged in parallel (increasing the cellular thickness), while in eccentric hypertrophy they are disposed in series (increasing the cellular length). Although the molecular basis of these responses is not well-known, two basic triggering mechanisms appear to be involved: neurohormonal and mechanical stretch activation (Fig. 19-1).
In neurohormonal activation associated with overload in vivo, the plasma concentrations of adrenergic agonists,
thyroxin, and angiotensin (Ang) increase. The activation of angiotensin II (Ang II) type 1 (AT1) and of α- and β-receptors by their endogenous agonists Ang II, adrenaline, and noradrenaline triggers the activation of the G-protein system, through metabolic pathways in which cyclic adenosine monophosphate (cAMP), Ca2+, phospholipase C, and other mediators, lead to the activation of protein kinases. These act by phosphorylating DNA binding proteins, reversing the inhibition that they exert over DNA transcription. This leads to expression of sequences coding for contractile proteins, ribosomal RNA, and oncogenes. Thus, protein synthesis and cell hypertrophy are initiated (4). Thyroid hormone levels are also increased as a consequence of neurohormonal activation. Thyroid hormone may act either through activation of the G-protein system, which triggers a similar response to that observed with Ang or α- or β-receptor agonists, or through activation of its receptor located in specific DNA sequences on target genes, which leads to the activation of contractile protein synthesis (3). In addition, both pressure and volume overload enhance mechanical stretch and cell deformation, thereby inducing activation of stretch-activated ion channels and G-proteins. This in turn activates protein kinase, which causes hypertrophy by the mechanisms previously outlined.
thyroxin, and angiotensin (Ang) increase. The activation of angiotensin II (Ang II) type 1 (AT1) and of α- and β-receptors by their endogenous agonists Ang II, adrenaline, and noradrenaline triggers the activation of the G-protein system, through metabolic pathways in which cyclic adenosine monophosphate (cAMP), Ca2+, phospholipase C, and other mediators, lead to the activation of protein kinases. These act by phosphorylating DNA binding proteins, reversing the inhibition that they exert over DNA transcription. This leads to expression of sequences coding for contractile proteins, ribosomal RNA, and oncogenes. Thus, protein synthesis and cell hypertrophy are initiated (4). Thyroid hormone levels are also increased as a consequence of neurohormonal activation. Thyroid hormone may act either through activation of the G-protein system, which triggers a similar response to that observed with Ang or α- or β-receptor agonists, or through activation of its receptor located in specific DNA sequences on target genes, which leads to the activation of contractile protein synthesis (3). In addition, both pressure and volume overload enhance mechanical stretch and cell deformation, thereby inducing activation of stretch-activated ion channels and G-proteins. This in turn activates protein kinase, which causes hypertrophy by the mechanisms previously outlined.
The consequence of these responses is enhanced metabolic activity and increase in the number of sarcomeres, which provide a short-term adaptation to overload. However, several of the growth factors that accelerate protein synthesis, including the proto-oncogenes c-myc and c-fos and the peptide transforming growth factor-β (TGF-β), have recently been shown to be capable of stimulating programmed cell death. This may be one of the mechanisms by which the unnatural growth triggered by overload also leads to accelerated myocyte death and impairment of contractility. Prevention of apoptosis may be one of the benefits of neurohormonal inhibition (5).
Quantification of Left Ventricular Performance in Patients with Chronic Valvular Disease
Evaluation of ventricular performance has important prognosis and therapeutic implications. Assessment of ventricular performance includes LV systolic function, volume, mass, LV geometry, LV diastolic function and pressure, and regional performance. Ventricular ejection fraction varies with heart rate, preload, afterload, and ventricular geometry even in the absence of changes in myocardial contractility.
Echocardiography is central to the diagnosis and follow-up of patients with valvular disease. Preoperative echocardiographic LV ejection fraction (LVEF) was the most powerful predictor of late survival in patients with chronic mitral regurgitation (MR) (6). According to American Society of Echocardiography (ASE) guidelines, echo evaluation of LVEF is best measured by biplane apical Simpson’s rule. However, this method has suboptimal reproducibility related to endocardial definition. Contrast cavity opacification markedly improves endocardial border delineation in patients with suboptimal image quality. A recent multicenter study showed that contrast echocardiography significantly improved interobserver agreement on LVEF compared with conventional echocardiography (7). Contrast echocardiography reaches a level comparable to magnetic resonance imaging (MRI) and is better than those obtained by cineventriculography (7). Other limitations of two-dimensional echo such as correct image plane
orientation and true long axis of the ventricle are overcome by three-dimensional echo. Contrast cavity opacification can be combined with three-dimensional echo for further improvement of LVEF accuracy and reproducibility. In addition, recently described indices of global LV systolic performance are not dependent on endocardial definition and are less dependent on loading conditions: dP/dt calculated from MR flow recordings, early diastolic mitral annular velocity, and peak systolic strain rate.
orientation and true long axis of the ventricle are overcome by three-dimensional echo. Contrast cavity opacification can be combined with three-dimensional echo for further improvement of LVEF accuracy and reproducibility. In addition, recently described indices of global LV systolic performance are not dependent on endocardial definition and are less dependent on loading conditions: dP/dt calculated from MR flow recordings, early diastolic mitral annular velocity, and peak systolic strain rate.
Ventricular volumes are also calculated with biplane apical Simpson’s rule. LV end-systolic volume seems to be less dependent on loading conditions and has been shown to be a strong prognostic factor and criterion for surgical indication in chronic aortic regurgitation (AR) and MR. Noninvasive hemodynamic assessment, including estimation of LV filling pressure, pulmonary artery systolic pressure (PASP), right atrial pressure, and cardiac output, can also be performed. Echocardiography is the investigation of choice to assess valve morphology, etiology, coexistence of other valvular disease, in addition to the changes in LV dimensions, wall thickness, and function (8.9). Quantification of valvular disease severity is obtained with the use of Doppler techniques. Multiplane transesophageal study is helpful when there is poor image quality with transthoracic echocardiography and to assess the mechanism and suitability for valve repair in mitral regurgitation. In addition, echo is practical for serial evaluations and guides the decision-making process for surgical referral. Transesophageal echocardiography is helpful to define valve anatomy, and mechanism of dysfunction, and is being used intraoperatively to assess results of mitral valve (MV) repair and for hemodynamic monitoring. History, physical examination, ECG, and exercise performance must also be taken into account.
During cardiac catheterization, direct measurement of transvalvular gradient, cardiac output, pressures in the cardiac chambers, ejection fraction, and LV dimensions can be performed. In patients with significant valve disease, cardiac catheterization, contrast ventriculography, and coronary angiography should be performed preoperatively when valve dysfunction is more than moderate, there is multiple valve disease, there are discrepancies between clinical and echo findings, and in patients who have angina or risk factors for coronary disease or who are older than 40 years of age (10,11).
Radionuclide angiography with gated pool imaging or first-pass angiography may be used to assess severity of regurgitation, LV volumes, and ejection fraction. MRI, when available, may be useful to assess the severity of valve regurgitation and ventricular volumes, particularly in patients with a poor ultrasonic acoustic window (12) when ultrasound contrast agents cannot be used and when the patient is not a transesophageal echocardiography candidate.
Aortic Stenosis with Left Ventricular Systolic Dysfunction
Etiology
In adults, degenerative or senile aortic stenosis (AS) has become the leading indication for valve replacement (13). In a large population above 65 years of age, advanced age, male sex, smoking, hypertension, height, and elevated lowdensity-lipoprotein (LDL) cholesterol were associated with AS or aortic sclerosis, suggesting that risk factors for AS or aortic sclerosis may be similar to risk factors for atherosclerosis (14). Degenerative disease associated with a bicuspid aortic valve accounts for 50% of cases of AS. Degenerative disease in a tricuspid aortic valve accounts for 12% of cases (15). In developed countries, rheumatic fever now accounts for fewer than 20% of cases of AS and is almost always associated with coexistent MV disease (16). Uncommon causes of AS in adults include atherosclerosis (in association with severe hypercholesterolemia and advanced atherosclerotic lesions in the aorta and great vessels), ochronosis, Paget bone disease, and advanced chronic renal failure.
Onset of Symptoms and Outcome
Symptoms appear late in the natural history of AS and are an ominous development (Fig. 19-2). The median untreated survival rates once angina pectoris or syncope has occurred are 5 and 3 years, respectively. HF occurs late in the natural history of the condition and is associated with the worst prognosis, the mean life expectancy then being less than 2 years (17). For patients with severe AS, the onset of the symptoms of angina, syncope, or CHF indicates the need for aortic valve replacement (17,18). The outcome is excellent after surgery in patients who have recently become symptomatic. Because sudden death can occur soon after the onset of symptoms, patients should be instructed to report changes in their clinical status. In cases in which the symptomatic status is unclear, supervised exercise testing may be helpful. The risk of exercise testing in these patients is lower than in those with classic symptoms of AS (19). If exercise performance is poor, surgical treatment should be considered (20). It should be noted that ST segment shifts during the exercise test may be related to LV hypertrophy.
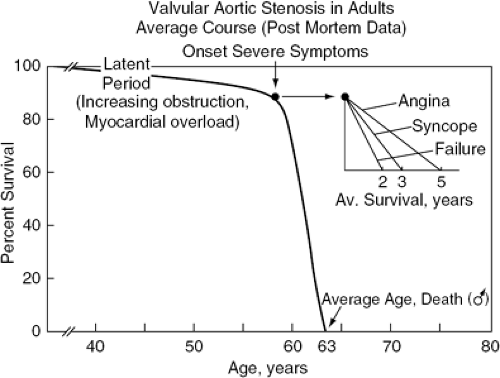 Figure 19-2 Natural history of AS. (Reproduced from Ross J, Jr., Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67 , with permission.) |
The rate of progression of AS from mild to severe forms has been shown to be quite variable between patients (21). In general, aortic area as calculated by the Gorlin formula decreases by about 0.1 cm2 per year (22,23) and Doppler-derived transvalvular gradient increases by 7 mm Hg per year (24). Therefore, mild AS may become severe within a few years. Several clinical features, including a degenerative etiology and the presence of risk factors for coronary disease, appear to be associated with more rapid progression, but no variable is predictive of the rate and pattern of progression in an individual patient (25).
Pathophysiology
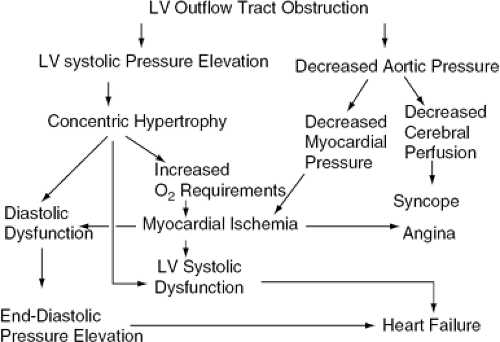
AS causes pressure overload (Fig. 19-3). Diastolic dysfunction precedes systolic dysfunction; thus, when congestive symptoms do arise, systolic function is usually normal. The hallmark adaptation to pressure overload is hypertrophy. This may be regarded as an attempt to normalize wall stress by increasing wall thickness. These adaptive phenomena parallel the progression of AS and help maintain systolic function within the normal range. In severe AS, however, the capacity to increase cardiac output during exercise may be impaired and end-systolic stress is not completely normalized (26). Furthermore, hypertrophy, which is associated with increased collagen deposition, causes increased LV stiffness resulting in reduced LV compliance and diastolic dysfunction (27). There is an upward shift of the pressure-volume relationship so that LV pressure is increased at any given diastolic volume. Elevated end-diastolic pressure may be transmitted to the pulmonary vascular bed, causing exertional congestive symptoms.
Patients with Aortic Stenosis, Low Cardiac Output, and Low Gradient
Some patients present with advanced CHF and reduced EF. Although reduced systolic function is usually an indicator of worse prognosis in most types of heart disease, it is less ominous for patients with AS. For those patients whose ejection performance is reduced because of the high afterload presented by the obstructing valve (afterload mismatch) and for whom the LV can still generate a mean Ao-LV gradient >30 mm Hg, prognosis is excellent following aortic valve replacement (26,28,29). In contrast, patients with AS, low gradient, and severe LV contractile dysfunction have a poor prognosis (28,29,30,31), with 21% operative risk and only 50% survival at 4 years (31). Nonetheless, many patients in this category do benefit from surgery (32).
Severe and calcified AS with low cardiac output is defined as aortic valve area <1 cm2 or <0.6 cm2/m2 with LV systolic dysfunction (LVEF <40%) and mean transvalvular gradient <40 mm Hg (33,34). Calculation of aortic valve area at rest cannot distinguish between patients with severe AS and patients with mild AS. In both cases, the calculated aortic valve area may be low (35,36,37). Surgical candidates can be selected using hemodynamic manipulation performed either in the echocardiographic or catheterization laboratories to distinguish between patients with severe valvular obstruction from those with only mild obstruction and aortic pseudostenosis (38,39).
Using echocardiography, patients with low EF and low gradient should be studied at rest and then following infusion of dobutamine. Dobutamine infusion starts at 5 μg/kg per minute and is progressively increased by 2.5-μg/kg/per/minute increments every 5 minutes and stopped when heart rate increase is ≥ 10 beats per minute. Therefore, inotropic effect is obtained with low risk of inducing ischemia (33). Noninvasive hemodynamic study with infusion of dobutamine detects aortic valve area changes during increase in stroke volume on the one hand, and also detects contractile reserve on the other hand. Contractile reserve with dobutamine stress is defined as 20% increase in stroke volume. Because the LV outflow tract diameter is unchanged, this is equivalent to a 20% increase in aortic velocity time integral (VTI). There are three possible responses to dobutamine stress (39):
Contractile reserve with increase in transvalvular gradient. Aortic valve area does not change or increases by less than 0.3 cm2 or to a valve area < 1.0 cm2, indicating the presence of truly severe aortic stenosis with preserved contractile reserve.
Contractile reserve without significant increase in transvalvular gradient. Aortic valve area increases by more than 0.3 cm2 or to a valve area >1.0 cm2, indicating nonischemic or ischemic cardiomyopathy with aortic pseudostenosis.
Using an invasive approach, nitroprusside can be infused during cardiac catheterization (38,41,42). If the stenotic valve is the primary and main obstruction to outflow, infusion of a vasodilator will decrease total peripheral resistance but flow cannot increase through the stenotic valve. There will be a fall in downstream pressure and an increase in gradient with little change in output. In this case, aortic valve area does not increase or may even decrease. A major risk in the use of sodium nitroprusside to make this determination is the potential for fall in downstream pressure in patients with true severe stenosis.
Thus, the drug must be used with caution, increasing the dose in tiny increments while monitoring hemodynamics carefully. On the other hand, if the valve is not severely stenotic (aortic pseudostenosis), then vasodilatation decreases total peripheral resistance, resulting in increased flow through the mildly stenotic valve; the gradient changes little and the valve area increases substantially. Coronary angiography can be performed during the same session.
Management
There is no major role for medical therapy in the management of AS. If the patient is asymptomatic, no treatment is required, and if the patient is symptomatic, surgery is indicated. Reduction of both preload and afterload can be detrimental and associated with hypotension and prerenal failure. For this reason, angiotensin-converting enzyme (ACE) inhibitors are generally contraindicated in AS and diuretics must be used with caution. In patients who are not surgical candidates, digoxin and diuretics may transiently relieve symptoms but no long-term benefit has been shown. Medications may also be of value in maintaining sinus rhythm in cases associated with atrial fibrillation.
Balloon aortic valvotomy is not indicated in adults because the rate of serious complications of the procedure exceeds 10% and the mortality 18 months after the procedure is similar to that observed in an untreated population. However, it may improve the clinical and hemodynamic status of very sick patients for a short time and serve as a bridge to aortic valve replacement in critically ill patients (43).
Aortic valve replacement remains the treatment of choice. Even octogenarian patients who are otherwise fit have been shown to do well following surgical treatment (44,45,46,47). When aortic valve replacement is indicated, there is increased risk of surgery if another valve has to be replaced or repaired, if coronary artery bypass grafts or aortic root replacement are required, and if the patient has renal failure or poor general condition. In patients with AS and low gradient the presence of a contractile reserve under dobutamine is a strong indicator of favorable outcome after aortic valve replacement. Although the absence of contractile reserve is not a contraindication for surgery, it should be combined with other operative risk factors to assess the risk-benefit ratio. In patients with aortic stenosis and low gradient, presence of contractile reserve under dobutamine and mean transvalvular gradient <20 mm Hg before aortic valve replacement were two independent prognostic factors of 30-day surgical mortality after aortic valve replacement (40). In addition, preserved contractile reserve was associated with symptomatic improvement and better long-term survival after aortic valve replacement.
Patient-prosthesis mismatch is responsible for higher mortality and poorer regression of LV hypertrophy when smaller valve sizes with larger gradients are inserted in patients with low LVEF (31,48,49,50). If the patient begins with only a 25-mm Hg transvalvular gradient, a residual gradient of 10 mm Hg represents 40% of the original gradient. Patient-prosthesis mismatch is defined by an effective orifice area ≤ 0.8 cm2/m2 (50,51). The risk of mismatch is real in patients with severe AS because they have a smaller aortic annular size due to the underlying pathological process. Tables indicating the size of each prosthetic valve according to body surface area (BSA) are useful. If the surgeon cannot insert an appropriate-sized prosthetic valve according to the reference tables, options include stentless bioprosthetic valves, new mechanical valves with best hemodynamic profile, homografts, the Ross procedure, and aortic root enlargement (52).
Chronic Aortic Regurgitation
Etiology
The causes of AR may be divided into those that affect the aortic valve and those that affect the aortic root. Of patients undergoing surgery, rheumatic disease has been shown to account for approximately 25%, endocarditis for 20%, and aortic root disease for 42% (53).
The prevalence of rheumatic AR is declining; it generally coexists with AS and rheumatic mitral disease (54). Infective endocarditis, which typically affects bicuspid, rheumatic, or otherwise structurally abnormal valves, often presents as an acute or subacute hemodynamic deterioration that requires rapid identification and management (Fig. 19-4). The regurgitant jet may impact and infect the anterior leaflet of the MV, worsening the natural history of the disease (55). Other valvular causes of aortic insufficiency include deterioration of a bioprosthetic valve, thoracic trauma causing cusp rupture (56), myxomatous degeneration, and collagen vascular diseases. Bicuspid aortic valves are predominantly associated with AS but in some cases AR may coexist or predominate. Other congenital diseases, such as ventricular septal defects, subaortic stenosis, and rupture of fenestrated aortic cusps, are rare causes of AR.
Conditions causing AR through dilatation of the aortic root are age-related or degenerative aortic dilatation, cystic medial necrosis of the aorta, Marfan syndrome, hypertension, syphilis, aortic dissection, ankylosing spondylitis, Behçet syndrome, Reiter disease, and Takayasu disease.
Pathophysiology
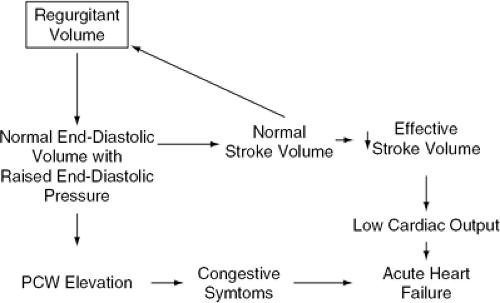
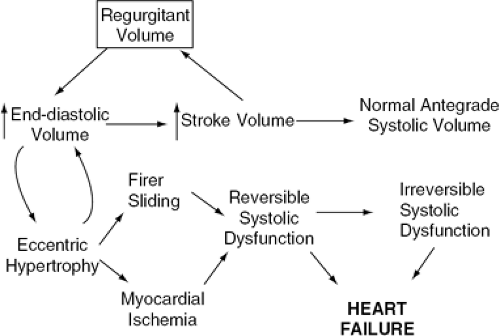
Chronic AR is responsible for combined volume and pressure overload of the LV (Fig. 19-5). However, if the AR progresses slowly, several adaptive phenomena occur, allowing cardiac output and LV end-diastolic pressure to remain within the normal range even during strenuous exercise (Fig. 19-6). Cardiac chamber dilatation is the most important of these adaptive changes. Dilatation of the LV and pericardium allow a rightward shift of the pressure-volume relationship, so that any given volume of blood can be accommodated at a lower pressure than in a normal-size chamber (57). As a consequence of the increased end-diastolic volume, an increase in stroke volume is achieved, mediated by a normal performance of each myocardial segment on an enlarged circumference (58). For this reason, although the regurgitant fraction can reach 50% to 80% of the total stroke volume, the effective antegrade stroke volume remains within normal range. Because the increase in ventricular diastolic volume is proportional to the increase in stroke volume, there is no change in EF. The increase in wall stress caused by increased ventricular volumes is accompanied by compensatory eccentric LV hypertrophy. In AR, the molecular mechanisms of hypertrophy are thought to be similar to those described for AS. The replication of sarcomeres is mainly in series, leading to dilatation as well as hypertrophy.
There is a transitional stage in which the LV progressively dilates and acquires a more spherical geometry, accompanied by increasing LV diastolic pressures and systolic wall stress. Subsequently, LVEF decreases, which eventually leads to symptoms of HF. These changes may be produced either by an increase in regurgitant fraction or by a decline in LV contractility related to fibrosis, sliding of muscle fibers, ischemia, or apoptosis (3,5). The progression of LV dysfunction is slow. In follow-up studies (59,60,61) of asymptomatic patients with severe AR, LV dilatation, and preserved EF, only 4% to 5% of patients per year needed surgical treatment.
Clinical Features
During the compensated phase, patients with chronic AR may remain asymptomatic for years. Symptoms of HF usually develop insidiously. The clinical features of chronic AR are described in classic texts. Infective endocarditis is the main cause of rapid worsening of AR.
Assessment
Echocardiography is the investigation of choice to assess the severity of AR, its etiology, and its effects on LV dimensions and function (8,9). In addition, it is of considerable importance in the follow-up of patients with chronic AR. Echocardiographic criteria for surgical referrals are discussed later. Three-dimensional echo provides refined structural evaluation of the valve.
During cardiac catheterization, the severity of AR may be assessed by characterizing the degree of LV opacifkation. LV diastolic pressure, resting cardiac output, and LVEF, end-systolic and end-diastolic diameters and volumes can be measured (11). The indications for cardiac catheterization are similar to those for AS.
Management
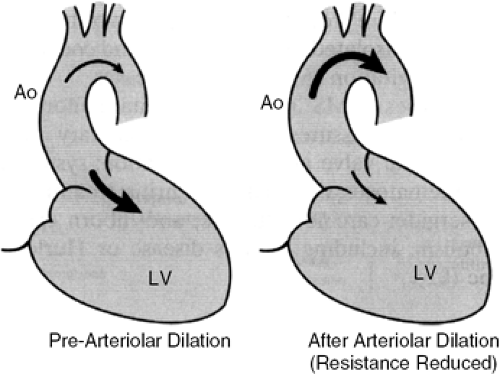
The distribution of blood flow in AR is sensitive to manipulations that alter the impedance to flow in the systemic circulation. Vasodilator therapy, by reducing afterload, may reduce the regurgitant fraction and LV end-diastolic volume and pressure (Figs. 19-7 and 19-8) (62,63,64,65,66,67).
Patients who are symptomatic or who have LV dysfunction should be referred for aortic valve replacement (68). Symptomatic patients should undergo surgery even in the presence of normal LV dimensions and function. Symptoms caused by AR are strong predictors of clinical prognosis. Patients with an EF of less than 50% at rest should undergo surgery (2). In a study of 246 patients with moderate to severe AR, the adverse event rate was 2.5% in 113 asymptomatic patients and 25% per year in symptomatic New York Heart Association (NYHA) Class III and IV patients (69). Independent predictors of survival were age, symptom severity, comorbidity, LV end-systolic diameter index, and atrial fibrillation. An initial conservative strategy
may be appropriate for patients with LV enlargement and preserved systolic function. Parameters associated with a poor postoperative prognosis are an end-systolic dimension greater than 55 mm (68) (Fig. 19-9); an end-systolic volume greater than 60 mL/m2, a shortening fraction below 28% (70), and ratio of LV end-systolic dimension to BSA greater than 26 mm/cm2 (71). Current ACC/AHA guidelines recommend valve replacement for patients with severe LV dilatation (end-diastolic dimension greater than 75 mm or end-systolic dimension greater than 55 mm) (2). These guidelines were published in 1998 and cut-off values of parameters are likely to decrease in the next version. Women undergoing surgery have a worse long-term postoperative outcome than do men and may warrant earlier intervention than is currently recommended (72). In cases of severe dysfunction (EF less than 30%), the operative mortality is high (reaching 10% or more), but overall prognosis is better than with conservative management (73). Duration of LV dysfunction has an impact on outcome. Frank LV dysfunction present for less than 1 year does not carry the same ominous prognosis as does a prolonged period of LV dysfunction (74,75).
may be appropriate for patients with LV enlargement and preserved systolic function. Parameters associated with a poor postoperative prognosis are an end-systolic dimension greater than 55 mm (68) (Fig. 19-9); an end-systolic volume greater than 60 mL/m2, a shortening fraction below 28% (70), and ratio of LV end-systolic dimension to BSA greater than 26 mm/cm2 (71). Current ACC/AHA guidelines recommend valve replacement for patients with severe LV dilatation (end-diastolic dimension greater than 75 mm or end-systolic dimension greater than 55 mm) (2). These guidelines were published in 1998 and cut-off values of parameters are likely to decrease in the next version. Women undergoing surgery have a worse long-term postoperative outcome than do men and may warrant earlier intervention than is currently recommended (72). In cases of severe dysfunction (EF less than 30%), the operative mortality is high (reaching 10% or more), but overall prognosis is better than with conservative management (73). Duration of LV dysfunction has an impact on outcome. Frank LV dysfunction present for less than 1 year does not carry the same ominous prognosis as does a prolonged period of LV dysfunction (74,75).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree