Pathophysiology of congestion. Abbreviations: RV right ventricular, RA right atrial, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, LA left atrial, LV left ventricular, LVDP left ventricular diastolic pressure, JVD jugular venous distension. (From Gheorghiade et al. [1])
Weight Gain
Weight gain has also been used as a surrogate for congestion. A rapid increase in weight can precede decompensated heart failure and greatly increases the risk for hospitalization in patients with heart failure. However, other factors can influence weight and not all weight gain is attributable to decompensated heart failure. In addition, a large number of patients who are hospitalized for decompensated heart failure have little or no weight gain [5, 6].
Clinical Profiles
Clinical profiles of congestion have been used to provide prognostic information and to guide therapy. Characterizing heart failure patients based on the clinical indicators of perfusion and congestion as either warm, cold, wet, or dry is relatively easy to do using information available in the history and physical examination. Patients described as warm and dry after treatment in the hospital have better clinical outcomes than patients with other clinical profiles [2]. While this framework is useful in the clinical setting, the clinical indicators of congestion are often inaccurate as described above.
Fluid Compartments
While increases in left-sided filling pressures can rapidly occur due to shifts in blood compartments (this occurs largely between the splanchnic venous beds and the effective arterial circulation), this scenario is generally not the primary process in patients with refractory congestion who might be considered candidates for ultrafiltration [7].
Blood Volume
Heart failure is a sodium avid state that often leads to expansion of total body water and total blood volume. For this reason, blood volume analysis using radiolabeled Iodine-131 dilution techniques can be used as another surrogate for congestion. Physical manifestations of congestion are not associated with total blood volume and increased blood volume is significantly associated with elevated left-sided filling pressures [8]. In one study, 65% of heart failure patients who were euvolemic by physical examination were actually hypervolemic when total blood volume was measured. In another study only 37% of patients hospitalized with decompensated heart failure had an increase in total blood volume [9]. While blood volume analysis introduces a more quantitative approach to the assessment of congestion, it is only a surrogate for elevated left-sided filling pressures – increases in blood volume only explain approximately half the variation in measured wedge pressure [8]. Blood volume analysis is rarely used clinically in part because it requires handling radioactive materials and multiple blood draws to create an accurate dilution curve and because its value in directing therapeutic decisions has not yet been demonstrated.
Persistent Congestion
Association of persistent congestion with clinical outcomes
Indicators of persistent congestion | Clinical correlates to persistent congestion | |
|---|---|---|
Lucas 2000 [10] | Scoring system including orthopnea, jugular venous pressure, change in weight, edema, and the need for IV diuretics 4–6 weeks after hospital discharge for heart failure | Increased mortality 2 years following hospital discharge |
Wattad 2015 [11] | Scoring system including jugular venous pressure, hepatomegaly, edema, rales, third heart sound | Increased mortality, mean follow up 15 months |
Aoki S [12] | Diuretic response expressed as weight loss/40 mg furosemide equivalent dose, edema, jugular venous distention | Increased cardiac death and rehospitalization for worsening HF 1 year after hospital discharge |
Lala A 2015 [13] | Orthodema score based on presence of orthopnea and degree of edema | Increased rates of death, rehospitalization, or emergency department visits 60 days after hospital discharge |
Kociol RD 2013 [14] | Weight loss, net fluid loss, reduction in NT Pro BNP | Increased rates of death, rehospitalization, or emergency department visits 60 days after hospital discharge |
Abraham 2011 [15] | Pulmonary artery pressure measured directly by wireless pulmonary artery monitoring system | Increased heart failure related hospitalizations 6 months after randomization |
Darawsha 2016 [16] | Scoring system including jugular venous pressure, hepatomegaly, edema, rales, third heart sound | Increased mortality, mean follow up 14 months |
Diuretics for the Management of Congestion
Diuretics are first-line therapy for patients with heart failure and congestion [13, 18]. The goal of therapy is to relieve congestion by increasing urine output and removing excess intravascular and extravascular fluid [1]. Loop diuretics such as furosemide exert their action on the thick ascending portion of the loop of Henle to block the sodium-potassium-chloride transporter [19]. This results in an increase in urinary excretion of sodium, chloride, calcium, magnesium, and potassium. In general, plasma water follows sodium in the nephron resulting in an increase in urine production. Diuretics can sometimes be challenging to use because the dose response between individuals can be highly variable and electrolytes must be closely monitored and replaced. In addition, diuretic resistance is common, often requiring increasing doses to achieve similar degrees of urine output [20].
Diuretics produce hypotonic urine and this reduces the effective removal of excess total body sodium present in patients with heart failure. As a result, these drugs are often ineffective. In one large registry of over 100,000 patients hospitalized with acute decompensated heart failure, more than 90% received intravenous (IV) diuretics yet nearly half failed to lose any weight after treatment with IV diuretics [21]. In a clinical trial of diuretic dosing strategies, clinical decongestion was achieved in less than 20% percent of patients regardless of whether patients received high dose or low-dose diuretics, intermittent boluses or continuous intravenous infusions [22]. Vasodilators, inotropes, and other agents have been added to diuretics in an attempt to preserve renal function and improve outcomes yet these efforts have failed [23–27].
Refractory Congestion
Definitions of refractory congestion
Ellison 2011 [28] | When moderate doses of diuretics fail to achieve the desired volume reduction |
Sackner-Bernstien 2003 [29] | A lack of response to 200 mg of furosemide per day |
Simpson 1986 [30] | Persistent edema despite treatment with diuretics, vasodilators, and inotropes |
Dormans 1996 [31] | Failure to lose weight or to develop a negative sodium balance despite bedrest, sodium restriction to <80 mmol/day, and high dose furosemide (> 250 mg/day) |
Bart 2012 [32] | Worsening renal function in the setting of IV diuretics with (a) pulmonary capillary wedge pressure >22 mm hg and at least 2+ peripheral edema and/or pulmonary edema or pleural effusions on chest x-ray; or (b) at least two of the following: ≥2+ peripheral edema, jugular venous pressure >10 mm Hg, and pulmonary edema or pleural effusions on chest x-ray |
ter Maaten 2015 [33] | (1) persistent congestion despite adequate and escalating doses of diuretic with >80 mg furosemide per day and/or (2) amount of sodium excreted as a percentage of fltered load less than 0.2% and/or (3) failure to excrete at least 90 mmol of sodium within 72 h of a 160 mg oral furosemide dose given twice daily |
Mentz 2014 [17] | Failure of diuretics to control volume status adequately despite appropriate dose escalation |
Ultrafiltration for the Management of Congestion
Ultrafiltration is the mechanical removal of isotonic plasma water directly from the circulation. Blood is withdrawn from a vein and flows across a semipermeable membrane under pressure to separate isotonic plasma water from blood. The plasma water is discarded and the remaining blood is returned to the patient [36]. Simplified ultrafiltration devices can be used in a variety of settings without the need for central venous access. Low blood flow rates are well tolerated even in patients with advanced heart failure and plasma water removal rates can be adjusted across a range from 0 to 500 mL/h. In contrast to diuretics which produces a hypotonic urine, ultrafiltration removes isotonic plasma water which can result in greater overall sodium removal – an important objective in treating heart failure [37]. Ultrafiltration results in rapid and predictable fluid removal, restores responsiveness to diuretics in patients with diuretic resistance, has no direct effect on serum electrolytes, and does not directly stimulate the neurohormonal system [38–41].
Direct Comparisons of Diuretics and Ultrafiltration
Randomized controlled trials comparing ultrafiltration to diuretic-based strategies have been performed in the modern era of heart failure treatment. These trials contribute to a growing database of experience that suggests that ultrafiltration may be superior to diuretic-based strategies in the management of patients who fail to adequately respond to loop diuretics.
RAPID [42]
The Relief for Acutely Fluid Overloaded Patients with Decompensated Congestive Heart Failure (RAPID-CHF) trial was the first randomized controlled trial comparing diuretic-based therapies to ultrafiltration in patients with acute decompensated heart failure using a simplified ultrafiltration circuit. This feasibility study randomized 40 patients hospitalized with decompensated heart failure to usual care with intravenous diuretics versus a single 8 h course of ultrafiltration performed within the first 24 h of hospitalization. There was a trend for improved weight loss in the ultrafiltration group at 24 h and significantly greater net fluid loss (4650 mL versus 2838 mL, P = 0.001). RAPID-CHF demonstrated that ultrafiltration is well tolerated in patients with acute decompensated heart failure and may be an alternative to diuretic therapy.
UNLOAD [39]
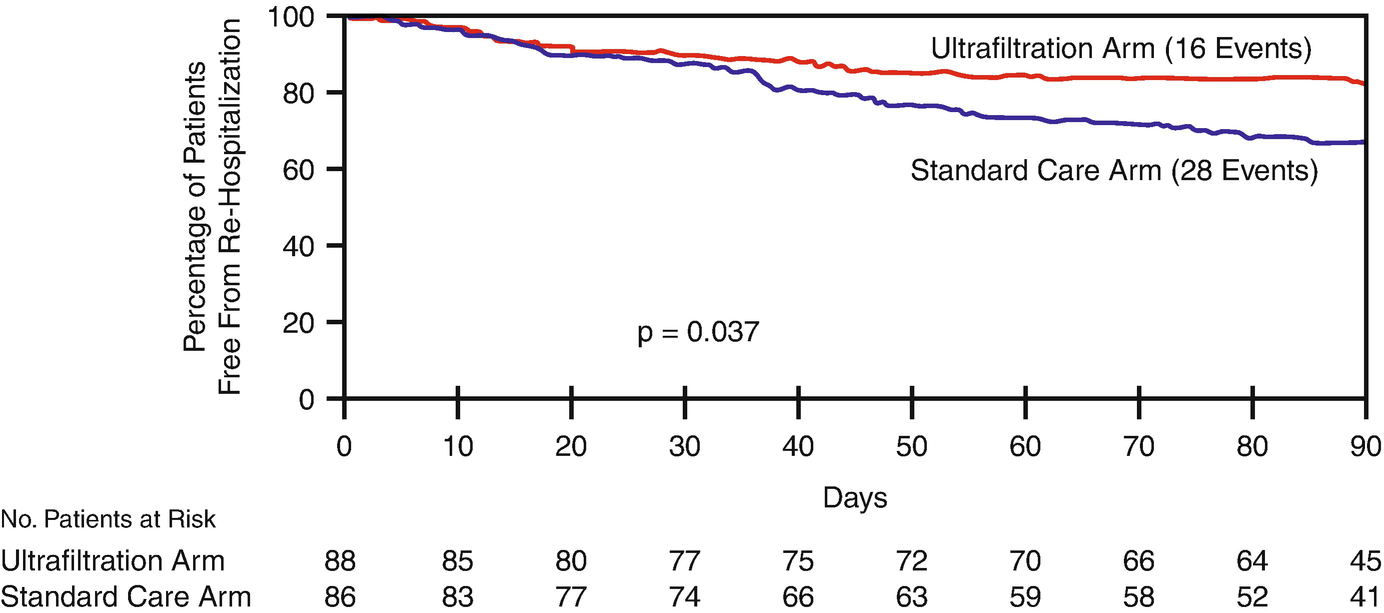
Freedom from heart failure rehospitalization. Kaplan-Meier estimate of freedom from rehospitalization for heart failure within 90 days after discharge in the ultrafiltration (red line) and standard care (blue line) groups. (From Costanzo [39])
ULTRADISCO [43]
The Effects of Ultrafiltration Versus Diuretics on Clinical, Biohumoral and Haemodynamic Variables in Patients With Decompensated Heart Failure (ULTRADISCO) study randomized 30 patients hospitalized with decompensated heart failure and congestion to a continuous IV infusion of furosemide versus ultrafiltration with a conventional renal replacement device using slow continuous ultrafiltration techniques. In the usual care group, the initial rate of furosemide infusion was 250 mg per 24 h and this dose was adjusted according to changes in creatinine, blood pressure, and heart rate. The dose was increased to 500 mg per 24 h if the initial dose did not achieve a negative fluid balance of at least 2000 mL per day. The ultrafiltration group initiated ultrafiltration with a fluid removal rate between 100–300 mL/h and this rate was adjusted according to blood pressure. Both groups achieved similar degrees of weight loss and fluid loss by the end of treatment. However, patients in the ultrafiltration group had significant improvements in cardiac performance when measured noninvasively using pulse contour analysis suggesting a possible advantage to ultrafiltration versus traditional diuretics.
Hanna, et al. [44]
This is the only randomized controlled study of ultrafiltration versus usual care in which all patients underwent invasive hemodynamic monitoring. Thirty-six patients, all with pulmonary arterial wedge pressure ≥24 mmHg were randomized to usual care with IV diuretics at the discretion of the treating physician or slow continuous ultrafiltration using a standard renal replacement device. The primary endpoint was the time required for the pulmonary arterial wedge pressure to fall ≤18 mmHg for at least four consecutive hours. Both groups experienced significant decreases in central venous pressure and pulmonary arterial wedge pressure and there was a trend favoring ultrafiltration for achieving the primary endpoint (22 h versus 34.8 h, P = 0.081). Despite more fluid removal in the ultrafiltration group (5213 mL versus 2167 mL, P = 0.041), there was no significant change in renal function. Length of hospital stay was lower in the ultrafiltration group (4.53 days versus 9.61 days, P < 0.001).
CUORE [45]
The Continuous Ultrafiltration for Congestive Heart Failure (CUORE) study randomized 56 patients hospitalized with decompensated heart failure and significant congestion to usual care involving IV diuretics (average dose of diuretics at initiation of therapy was 153 mg per day) or ultrafiltration for an average of 19 h with a mean plasma water removal of 4254 mL. Interestingly, diuretics were continued in patients randomized to the ultrafiltration group. There was no significant difference in weight loss achieved in the two groups (7.5 kg for ultrafiltration versus 7.9 kg for usual care, P = 0.75). There was no difference in length of hospital stay. However, 6 months after discharge, patients in the usual care group gained more weight, required higher doses of diuretics, and had higher creatinine compared to the ultrafiltration group. Ultrafiltration patients had fewer heart failure readmissions after 12 months of follow-up compared to usual care (hazard ratio 0.14, P = 0.002).
CARRESS-HF [32]
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) randomized 188 patients with acute kidney injury and persistent congestion after failing standard treatment with escalating doses of diuretics to a diuretic-based, stepped pharmacologic care treatment protocol designed to achieve 3–5 L of urine output per day or ultrafiltration (average treatment duration 40 h, target plasma water removal rate of 200 mL/h). The primary endpoint was change in weight and change in creatinine measured 96 h after randomization. There was no significant difference in weight loss and a transient increase in creatinine at 96 h which resolved 30 days after discharge. There were no differences in clinical outcomes at 60 days. Due to a significant number of dropouts and crossovers from the ultrafiltration arm of the trial to the diuretic-based arm of the trial, an analysis was performed comparing subjects who actually received their assigned treatment after randomization. In this per-protocol analysis, patients receiving ultrafiltration had significantly greater net fluid loss and weight reduction than patients receiving pharmacologic therapy [46] Ultrafiltration was associated with higher creatinine and blood urea nitrogen values, lower serum sodium concentrations, and increased plasma renin activity; pharmacologic therapy was associated with higher serum bicarbonate. However, there were no significant differences in 60-day outcomes suggesting that transient increases in serum creatinine associated with ultrafiltration are not clinically significant [46].
AVOID-HF [47]

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


