Congenital Heart Disease in Adults
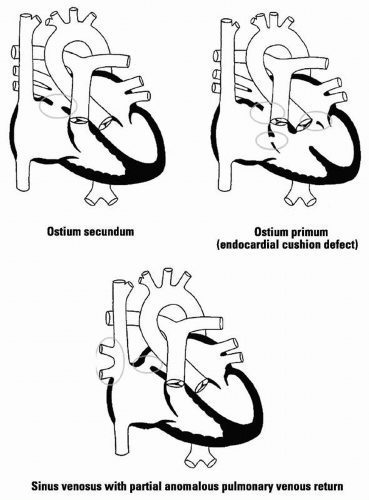
15.1 Atrial Septal Defect
Nejm 2000;342:256
Cause:
ASD: 75% ostium secundum defects (fossa ovalis); 15% ostium primum defects (lower intra-atrial septum); 10% sinus venosus defects (upper intra-atrial septum); most often due to spontaneous mutations
10-20% ofpts with secundum ASD are reported to have mitral valve prolapse (Am J Cardiol 1976;38:167).
Lutembacher’s syndrome: ASD plus (rheumatic) mitral stenosis
Epidem: The population of U.S. adults with surgically corrected or uncorrected congenital heart disease is increasing 5%/yr. ASD represents 1/3 of all cases of congenital heart disease detected in adults; it is 2-3 times more common in women.
AV canal defects (endocardial cushion defects) are seen with trisomy 21 (Down’s syndrome).
Pathophys: A shunt from the LA to RA produces increased pulmonary blood flow and dilatation of the RA, RV, pulmonary arteries, and LA; defects < 0.5 cm in diameter are usually not hemodynamically significant. Mitral valve prolapse is associated with ostium secundum defects; cleft anterior mitral leaflet is seen
with ostium primum defects. Partial anomalous drainage of pulmonary veins can accompany sinus venosus defects.
with ostium primum defects. Partial anomalous drainage of pulmonary veins can accompany sinus venosus defects.
Sx: Mostpts asymptomatic for years; exertional dyspnea; fatigue; palpitations, paradoxical emboli likely after age 60
Si: S1 normal; fixed splitting of S2 (phasic changes in venous return to RA with respiration matched by changes in volume of LA to RA shunt), soft mid-systolic ejection murmur (pulmonary valve) or diastolic flow murmur across tricuspid valve may also be present
Pansystolic murmur of mitral regurgitation present with cleft mitral valve accompanying ostium primum defect
Crs: Pts with secundum ASD survive into adulthood but have shortened life expectancy (50% survival past age 40). Those with large shunts die of RV failure or arrhythmia in the 4th-5th decades.
Pts with secundum or sinus venosus ASD who are minimally symptomatic in the 3rd decade do not develop severe pulmonary HT and do well with medical management (Brit Hrt J 1994; 71:224).
Symptomaticpts have near-normal life expectancy and morbidity when surgery is performed before age 25 (Nejm 1990;323: 1645). Surgery in olderpts improves survival but not the incidence of atrial arrhythmias (which are related to atrial dilation) and thromboembolic events compared with medical rx (Nejm 1995;333:469).
Cmplc: Pts develop at least mild/moderate pulmonary HT after age 40; 10-15% develop severe pulmonary HT with shunt reversal and resultant cyanosis. 15% ofpts develop mitral fibrosis and mitral regurgitation.
Lab:
Echocardiogram: Dilated atria and RV; ostium primum/secundum defects may be visible directly or detected via contrast study;
TEE superior for detection of ASD and anomalous pulmonary venous drainage
TEE superior for detection of ASD and anomalous pulmonary venous drainage
Cardiac catheterization: To measure magnitude of shunt and presence of pulmonary HT and for detection of CAD in olderpts
X-ray: CXR: Prominent pulmonary arteries with peripheral pulmonary vascular pattern well defined (“shunt vasculature” pattern); anomalous pulmonary veins may be visible
Rx: Surgical closure for pulmonary/systemic flow ≥ 1.8; surgery not recommended forpts with irreversible pulmonary vascular disease and pulmonary HT; endocarditis prophylaxis not recommended unless valve disease also present
Pts with ostium primum ASD and cleft mitral valve may require valve repair/replacement
15.2 Ventricular Septal Defect
Nejm 2000;342:256
Cause: 70% of VSDs are located in membranous septum; 20% in the muscular septum; 5% below aortic valve and cause AI; 5% near mitral/tricuspid valves (AV canal defects)
Epidem: Adult survivors either have had spontaneous closure/near closure or develop increased pulmonary vascular resistance with systemic right-sided pressures and relief of persistent LV volume overload (Eisenmenger’s syndrome)
Pathophys: VSD initially produces shunt from LV to RV. With large defects, pressures eventually equalize or reverse as pulmonary HT worsens.
Sx: Cyanosis with R-to-L shunting; decreased exercise tolerance and DOE; palpitations (due to Aflut or Afib); visual disturbances, fatigue, headache, dizziness, paresthesias if hyperviscosity syndrome develops with Eisenmenger’s syndrome. Adults with a shunt ratio < 2:1 are usually asymptomatic; with a ratio of 3:1, exertional dyspnea is likely after age 30.
Si: Cyanosis; clubbing; normal/split S2; S3; holosystolic murmur loudest at lower LSB ± palpable thrill with restrictive defect; mid-diastolic rumble; murmur of AI if VSD near aortic annulus; with muscular VSD, murmur may end in mid/late systole as muscle contracts; murmur diminishes/disappears as flow decreases; murmur of pulmonary regurgitation (Graham Steell murmur) may appear
Crs: 25-40% close spontaneously by age 2; 90% of defects that close do so by age 10. Adults with small defects are usually asymptomatic and unlikely to develop pulmonary HT. In adults diagnosed with VSD, 10-yr survival ≈75%. Those presenting with cardiomegaly, pulmonary HT, or impaired exercise capacity have poorer prognosis.
Cmplc: Even a small VSD still carries an increased risk for infective endocarditis. With a large shunt, development of Eisenmenger’s syndrome is associated with risk of hemoptysis due to pulmonary infarction or rupture of dilated pulmonary arteries/arterioles. CVA may result from paradoxical embolization, venous thrombosis of cerebral vessels, or intracranial hemorrhage. Brain abscess, syncope, and sudden death have also been reported.
Lab:
Echocardiogram: VSD may be visible directly or detected via contrast study or TEE; Doppler study can usually provide an estimate of PA systolic pressure
Cardiac catheterization: To measure magnitude of shunt and presence of pulmonary HT
X-ray: CXR: Normal with small VSD; LV enlargement and “shunt vascularity” with large defect; enlargement of proximal pulmonary arteries, rapid tapering of peripheral pulmonary arteries, and oligemic lung fields with pulmonary HT
Rx: Small VSD requires antibiotic prophylaxis for infective endocarditis; adults with large defects will have CHF and pulmonary HT, and should have surgical closure if pulmonary/systemic vascular resistance < 0.7, QP:QS > 1.5:1
At 25 yr after surgery, the cumulative incidence of infective endocarditis is 2.7% inpts with isolated VSD.
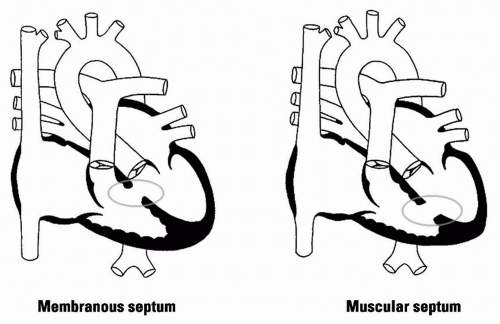 Figure 15.2 VSD |
15.3 Patent Ductus Arteriosus
Cause: Failure of closure of ductus arteriosus (connects proximal descending aorta to main PA bifurcation or left pulmonary artery distal to the left subclavian artery). In a fetus, pulmonary arterial blood flows into the descending aorta for oxygenation in the placenta; failure to close produces a L-to-R shunt from the aorta to the pulmonary artery.
Epidem: PDA accounts for ˜10% cases of congenital heart disease. Increased incidence is noted in premature infants or in pregnancies complicated by persistent perinatal hypoxemia or maternal rubella infection.
Pathophys: In the absence of increased pulmonary vascular resistance, blood is shunted from the aorta to the pulmonary artery throughout the cardiac cycle, producing left-sided volume overload.
Sx: Small PDA causes no sx. If sx develop, they usually do so in the 1st year of life. Fatigue, dyspnea, or palpitations are the most common sx reported.
Si: With moderate/large shunt: Bounding peripheral pulses and widened pulse pressure; hyperdynamic LV impulse; “machinery” murmur in 2nd LICS may obscure S2 and continues into diastole but shortens/disappears as pulmonary HT develops; flow murmurs across mitral and aortic valves; pulmonary ejection click and diastolic murmur of pulmonary regurgitation with pulmonary HT
Development of Eisenmenger’s physiology can produce “differential cyanosis” and clubbing of toes but not of fingers.
Crs: Spontaneous closure of PDA after infancy is rare; normal life expectancy with small defect, but increased risk of infective endocarditis (pulmonary side) and/or septic pulmonary emboli; LV failure, pulmonary vascular obstruction, and severe pulmonary HT; aneurysmal dilation, calcification, rupture of PDA may also occur
Cmplc: Risk of infective endocarditis exceeds that of CHF by 2nd decade; risk of endarteritis ≈ 0.45% annually after age 20 ˜1/3 ofpts with unrepaired PDA die from CHF, pulmonary HT, or endarteritis by age 40; 2/3 by age 60
Lab:
Echocardiogram: Ductus arteriosus occasionally visualized; continuous and increased flow in the pulmonary trunk seen on Doppler
Cardiac catheterization: To demonstrate ductus, quantify shunt, and allow calculation of pulmonary vascular resistance
X-ray: CXR: Normal with small PDA; large shunt produces dilated LA and LV, pulmonary vascular engorgement, dilation of proximal PA; prominent ascending aorta; ductus may be seen as an opacity at junction of aortic knob and descending aorta
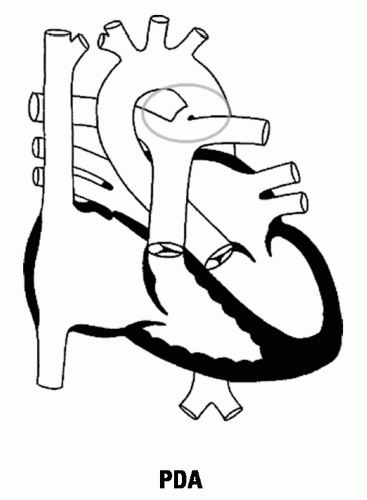 Figure 15.3 PDA |
Rx: Perioperative mortality for ligation of PDA < 0.5% without cardiopulmonary bypass. Surgical ligation or percutaneous closure is contraindicated if severe pulmonary vascular obstructive disease has developed.
Small PDA may be able to be closed with an intravascular device, but data on long-term follow-up are still pending.
Closure of a PDA eliminates the risk of infective endocarditis.
15.4 Congenital Aortic Stenosis
Cause: Bicuspid aortic valve is the most common pathological finding inpts with symptomatic AS < age 65. The valve has a single fused commissure and an eccentrically oriented orifice (Circ 2002;106:900).
Supravalvular stenosis and discrete subvalular aortic stenosis are also seen in childhood.
Epidem: Congenital bicuspid valve is found in 2-3% of the population. It is four times more common in males than in females.
20% ofpts with a bicuspid aortic valve have an associated cardiovascular abnormality (PDA, coarctation of aorta).
Pathophys: Bicuspid valve is subject to abnormal hemodynamic stress, which leads to thickening and calcification of leaflets. Coexisting conditions (fibrillin-1 and endothelial NOS gene abnormalities) predispose to dilatation of aortic root.
Si: See aortic stenosis: With severe AS, delayed carotid; decreased S2; S4 gallop; aortic ejection click disappears as valve becomes calcified; harsh late systolic murmur; aortic regurgitation common with subaortic stenosis
Crs: Asymptomatic adults have normal life expectancy. AS becomes hemodynamically significant when the valve area is reduced to ˜1 sq cm. Median survival is 5 yr after angina develops, 3 yr after syncope occurs, 2 yr after CHF develops.
Cmplc: Pts with bicuspid aortic valve have an increased incidence of cystic medial necrosis of the aortic root with aneurysm formation and aortic regurgitation or aortic dissection (J Am Coll Cardiol 1992;19:283).
Lab:
Echocardiogram: Will show aortic stenosis; Doppler allows assessment of valve area; TEE is superior in dx of subaortic and supravalvular stenosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree