, Domenico Corrado2 and Cristina Basso1
(1)
Cardiovascular Pathology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
(2)
Cardiology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
The natural history of some congenital heart diseases can be featured by electrical instability at risk of SCD, both at rest and on effort.
By definition, congenital heart disease is a structural defect present at birth. As such, genetically determined cardiomyopathies, like hypertrophic and arrhythmogenic, in which the phenotypic expression occurs in childhood, should not strictly be regarded as congenital heart diseases. They have been treated and illustrated separately under the chapter of cardiomyopathies.
The most life-threatening congenital heart diseases, at risk of SCD, are hidden and poorly symptomatic structural defects, quite difficult to detect at physical examination. This is the case of bicuspid aortic valve, congenital coronary artery anomalies with origin still from the aorta, but in the wrong sinus, and ventricular preexcitation due to AV accessory pathways (Wolff–Parkinson–White syndrome) [1]. These congenital malformations have been already discussed and illustrated within the chapters of valve, coronary, and conduction system disease, respectively.
Among overt congenital heart diseases, those with septal defects complicated by pulmonary vascular disease (Eisenmenger syndrome) have been reported in SCD series. SCD is indeed one of the modes of death in these patients (Figs. 8.1 and 8.2) [2].
The most electrically vulnerable hearts of subjects affected by congenital malformations are those which had undergone surgical repair. Injury of the AV conduction system during operation and/or myocardial scars following ventriculotomy or implantation of a conduit to reconstruct the continuity between the right ventricle and the pulmonary artery, like in tetralogy of Fallot, double-outlet right ventricle, truncus arteriosus, and transposition with pulmonary stenosis, are the usual arrhythmogenic substrates [1].
In complete transposition of the great arteries, atrial switch operation may also jeopardize the electrical cardiac stability with resection of the atrial septum [3]. Arterial switch repair, with reimplantation of the coronary arteries, has also sequelae in the long-term follow-up, when complicated by coronary artery stenosis at risk of ischemia and cardiac arrest [4, 5].
However, tetralogy of Fallot is the most frequently operated congenital heart disease at risk of SCD (Fig. 8.3). The arrhythmogenic substrate is a combination of right ventricular outflow tract fibrosis, because of infundibulectomy due to the closure of the ventricular septal defect and removal of subpulmonary stenosis, and of the implantation of a transannular patch for pulmonary outflow enlargement or pulmonary conduit [6–10]. Postoperative pulmonary valve incompetence may account for the right ventricular dilatation, which also contributes, together with the right ventricular myocardial fibrosis, to impair the electrical impulse transmission within the right ventricle and trigger reentrant life-threatening arrhythmias.
Finally, corrected transposition, in which the AV conduction system is congenitally displaced anteriorly along with the pulmonary outflow tract, is at risk of SCD because of congenital or acquired AV block (Figs. 8.4 and 8.5) [11, 12].
The increasing number of adult patients with congenital heart diseases (grown-up congenital heart disease-GUCH population), either successfully operated on or not, raises the need to control their cardiac electrical vulnerability [13–19]. Surgeons nowadays are fully aware of the need to avoid damaging the conduction system as well as limiting ventriculotomy only when strictly necessary. Both isolated ventricular septal defects and tetralogy of Fallot are nowadays repaired through an atrial approach, to avoid the risk of ventricular myocardial scarring.
8.1 Image Gallery
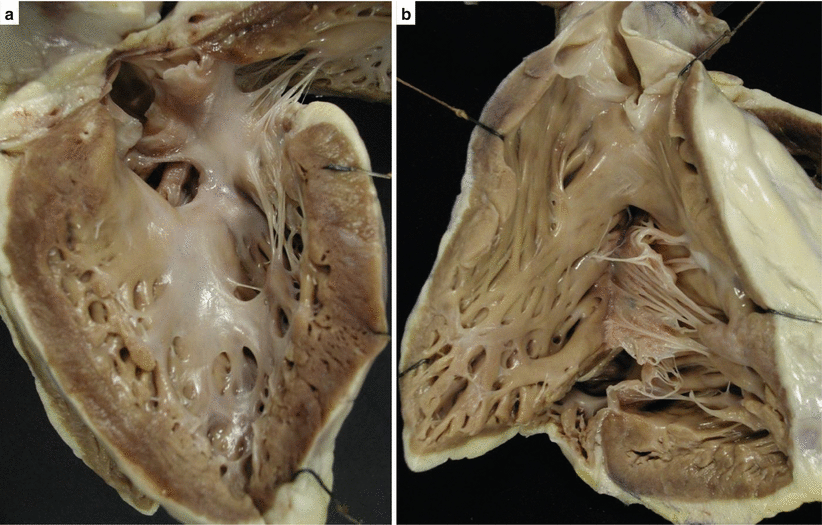
Fig. 8.1
Arrhythmic sudden cardiac death in 18-year-old boy with Eisenmenger complex. (a) Gross view of the perimembranous ventricular septal defect seen from the left side. The aorta is overriding the ventricular septum. (b) View of the right ventricular outflow tract with partial origin of the aorta from the right ventricle
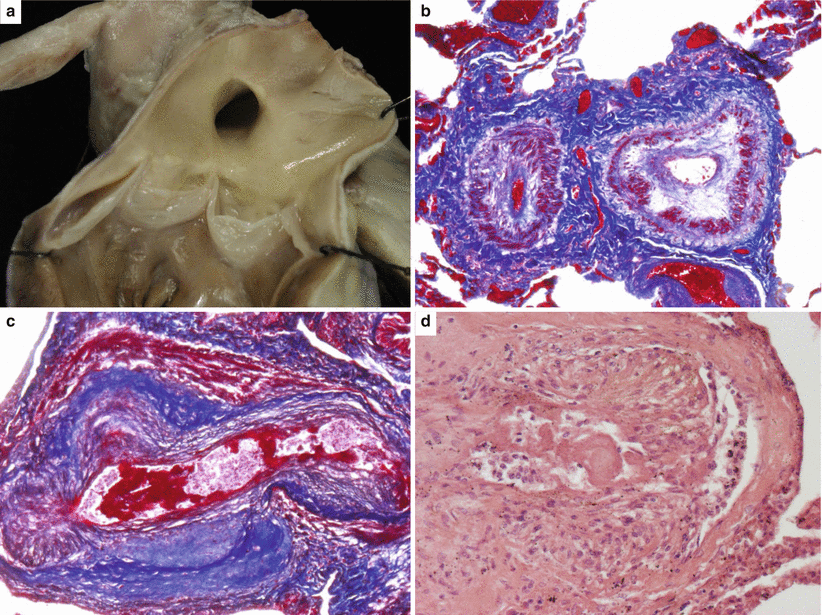
Fig. 8.2
Same case as Fig. 8.1. (a) Gross view of the pulmonary artery with atherosclerotic plaques in keeping with pulmonary hypertension. (b) Histology of the lung: small pulmonary arteries with obstructive intimal proliferation (Heidenhain trichrome). (c) Plexiform lesion with aneurysms of a small pulmonary artery (Heidenhain trichrome). (d) Glomoid proliferation and fibrinoid necrosis (hematoxylin–eosin)
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


