Figure 16.1 – Development of the heart and its chambers.
// PRO-TIP //
The atrial septum begins as the septum primum (ridge of tissue that grows downwards from the common atria). This leaves a large opening known as the ostium primum, which narrows as the septum primum grows. There is also a second opening known as the ostium secundum, which eventually closes up as the septum secundum grows. An opening, known as the foramen ovale, is left and remains open in utero.
16.1.2 Fetal circulation
- The fetus is still reliant on the maternal circulation for its oxygen, as it still has immature, under-developed lungs.
- Oxygenated blood is carried from the placenta to the right side of the heart via the following route: placenta → umbilical vein → ductus venosus → inferior vena cava → right atrium.
- From the right atrium, the oxygenated blood can take three different routes:
(i) via the foramen ovale: This makes up majority of blood flow. Blood travels from right atrium → foramen ovale → left atrium → left ventricle → aorta.
(ii) via the ductus arteriosus: this makes up most of the remaining blood flow. Blood flows from right atrium → right ventricle → pulmonary artery → ductus arteriosus → aorta. Blood flows through the ductus arteriosus instead of the lungs because the systemic vascular resistance (SVR) has a lower resistance than the pulmonary vascular resistance (PVR).
(iii) via the fetal lungs: only a tiny amount of blood is left to circulate through the immature lungs.
- After birth, the placenta no longer supplies blood to the baby. As the baby takes its first breath, the PVR drops significantly lower than the SVR. In contrast to (ii) above, blood now experiences minimal resistance from the pulmonary vasculature, and thus starts flowing through the lungs.
- The ductus arteriosus will close and form the remnant ligamentum arteriosum.
- The entry of blood into the left atrium through the pulmonary veins will also push the floppy septum primum up against the septum secundum, thereby closing the foramen ovale. This forms the fossa ovalis.
- In utero, left and right heart chamber pressures are equal. After birth, right-sided pressure gradually falls over several weeks due to the fall in PVR.
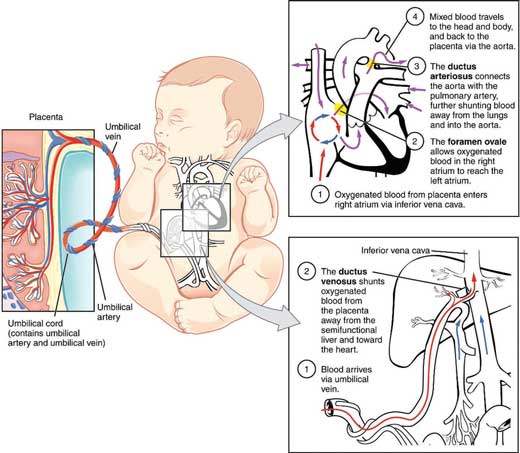
Figure 16.2 – The fetal circulation.
16.1.3 Epidemiology
- CHD (ranging from minor haemodynamically insufficient lesions to complex, severe congenital heart disease) is very common: about 1% of all births
- People of East Asian descent (Japan, China, etc.) have a higher rate of CHD
- Ventricular septal defects (VSD) are the most common CHD (30%).
16.1.4 Aetiology
There is no known aetiology for CHD, but there are important associations to be aware of:
- Down’s syndrome
- Maternal alcoholism and smoking.
Others
- Preterm infants
- Familial: a family history of congenital heart disease in 1st-degree relatives increases the risk from 1% to 4%
- Maternal factors: pregnant mothers with poorly controlled diabetes, hypertension, rubella infection or systemic lupus erythematosus (SLE) have a higher risk of having a baby with CHD
- Marfan’s syndrome, Di George syndrome
- Teratogenic drugs: e.g. lithium (specifically Ebstein’s anomaly, a CHD with displacement of the tricuspid valve and atrialisation of the right ventricle), certain anticonvulsants and ACE inhibitors. Warfarin may predispose to an atrial septal defect or a patent ductus arteriosus.
// PRO-TIP //
Down’s syndrome: occurs as a result of trisomy 21. Features include learning difficulties, epicanthic folds and a single palmar crease. 50% of children with Down’s syndrome will have a CHD, half of which are atrioventricular septal defects (AVSD).
Di George syndrome: occurs as a result of 22q11 deletion. Features include learning difficulties, cleft palate, hypoparathyroidism, bone and muscle deformities. Children with Di George syndrome suffer from a wide range of CHDs, including tetralogy of Fallot and persistent truncus arteriosus.
Marfan’s syndrome: occurs as a result of a mutation in a gene coding for fibrillin, a protein. Features include arachnodactyly, tall stature, lens dislocation. Aortic root dilatation, resulting in aortic regurgitation, is commonly seen.
Noonan syndrome: an autosomal dominant disorder involving a mutation in the ras family of genes. Features include short stature, learning difficulties, webbed neck and coagulopathy. Pulmonary stenosis, ASD and hypertrophic cardiomyopathy are commonly seen.
Edwards’ syndrome: occurs as a result of trisomy 18. Features include organ malformation, omphalocoele, cranio-facial abnormalities and severe learning difficulties. A ventricular septal defect, atrial septal defect and a patent ductus arteriosus are commonly seen.
Turner’s syndrome: Occurs as a result of missing an X chromosome (i.e. 45XO). Features include short stature, infertility (primary amenorrhoea), webbed neck and learning difficulties. A bicuspid aortic valve and coarctation of the aorta occur in 25% of children.
16.1.5 Clinical features
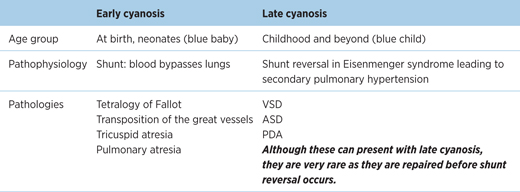
Table 16.1 – Early cyanosis versus late cyanosis
EXAM | The ‘CHAMP’ mnemonic is useful for general clinical features of CHDs. However, not every feature is present in each congenital defect. |
Cyanosis
- Due to deoxygenated blood entering the systemic circulation
- Can lead to finger and toe clubbing if prolonged.
Heart failure
- Occurs as a result of:
- unrestricted left-to-right shunt in large VSD, PDA, but not ASD
- obstruction that occurs as a result of coarctation of the aorta leads to left ventricular impairment
- unrestricted left-to-right shunt in large VSD, PDA, but not ASD
- Symptoms:
- poor feeding, associated sweating with feeds in young children
- decreased exercise tolerance
- breathlessness
- fatigue
- faltering growth: including growth restriction and/or mild learning difficulties.
- poor feeding, associated sweating with feeds in young children
Arrhythmia
- The type of arrhythmia is dependent on the pathophysiology e.g. AF secondary to atrial enlargement in atrial septal defects
- Paediatric ECGs are different from adults and require additional training for interpretation
- Most commonly associated with supraventricular tachycardia (SVT), including Wolff–Parkinson–White (WPW) syndrome. Some arrhythmias such as long QT and Brugada syndrome can present with syncope or sudden death.
Murmur
- Common in children and babies, majority as ‘innocent heart murmurs’
- Can be a result of turbulent flow across congenital defects
- Presentation is dependent on site of anatomical defect – e.g. a continuous machinery murmur in patent ductus arteriosus, a pansystolic murmur heard in VSD and a murmur heard upon auscultating the back from coarctation of the aorta.
Pulmonary hypertension
- In large left-to-right shunts under high pressure (e.g. VSD, PDA), the lungs are subjected to unrestricted increase in high pressure blood flow over time, which in the long term can lead to increased pulmonary resistance and pulmonary hypertension.
16.1.6 Pregnancy and CHDs
Women with complex CHD who are planning on getting pregnant are, despite treatment, at higher risk of complications during pregnancy.
As such, these patients should undergo specialist assessment to plan their care. In individuals with severe CHDs (complication rate >10%), it may be advisable to avoid pregnancy (contraceptive control) altogether. Fetal echocardiography should be performed during the 26th week of the pregnancy to exclude fetal abnormality.
// Why? //
During pregnancy, there is an increase in plasma and whole blood volume to accommodate the growing fetus. Where previously only a small amount of blood was shunted through the defect (for instance, an atrial septal defect), a much larger volume of shunted blood is now involved. Hence, the symptoms discussed above will become more apparent.
GUIDELINES: Congenital heart disease and pregnancy (NICE, 2008 and RCOG, 2011)
• Women with CHD should give birth in hospitals, supported by a maternity team
• 400 μg of folic acid a day recommended during the first trimester (first 12 weeks) of pregnancy. This lowers incidences of CHD among other birth defects.

Figure 16.3 – Progression of Eisenmenger’s syndrome.
16.1.7 Shunt reversal – Eisenmenger’s syndrome
Pulmonary blood flow that occurs as a result of a left-to-right shunt (caused by a septal defect) can progressively lead to increased pulmonary artery pressures due to the increased resistance of blood flow in the lungs, resulting in pulmonary hypertension. The pulmonary artery pressure and its corresponding right ventricular muscle mass may then eventually become greater than the left-sided pressure, causing a reversal of the shunt from right to left. Irreversible pulmonary hypertension can develop from 12 months of age and if progressive, may lead to Eisenmenger’s syndrome.
16.2 Patent ductus arteriosus (PDA)
PDA In A Heartbeat
Epidemiology | 10% of all CHDs |
Clinical features | Usually asymptomatic if small |
Investigations | Echo (gold standard) |
Management | Premature neonates: indomethacin/ibuprofen |
16.2.1 Definition
Failure of closure of ductus arteriosus.
16.2.2 Epidemiology
- This condition accounts for 10% of all CHDs
- More common in premature infants.
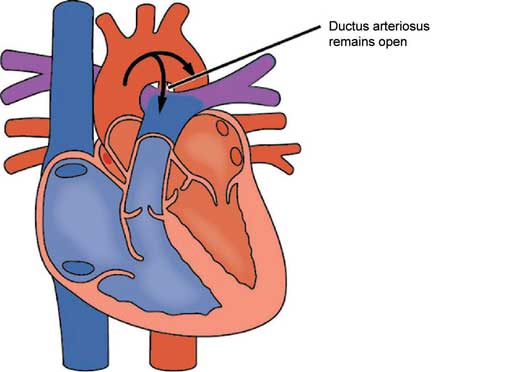
Figure 16.4 – Patent ductus arteriosus.
16.2.3 Pathophysiology
See Section 16.1.2 for essential prior knowledge.
Normally, the ductus arteriosus closes within 12–18 hours after birth but sometimes fails to do so or reopens – resulting in a patent ductus arteriosus (PDA).
// Why? //
The ductus arteriosus is provoked to close after birth by a rise in blood oxygen tension and reduced prostaglandins.
After birth, the blood from the aorta (higher pressure) is shunted into the pulmonary artery (lower pressure). If the shunt is small, it may be of no haemodynamic consequence. However, in a large shunt, this can result in excessive pulmonary blood flow and increased work for the left side of the heart.
// PRO-TIP //
PDA can, rarely, be caused by maternal rubella infection, in conjunction with other abnormalities including cataracts and microcephaly. This is known as the rubella syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


