Congenital Heart Disease
Thomas K. Kurian
Mohammed K. Saghir
Daniel H. Cooper
In recognizing the complexity of this topic, this chapter is intended to give an introduction to congenital abnormalities that may be seen in the adult population. Many of the patients will already have had some form of palliative or corrective surgery. In this regard, it is critical to obtain a thorough history of prior evaluation and treatment, as this will significantly impact the focus of image acquisition and interpretation.
Basic echocardiographic approach for evaluating unknown congenital heart disease (adapted from Diagnosis and Management of Adult Congenital Heart Disease by Gatzoulis):
Establish apex position: Using a standard subcostal view (probe indicator points to patient’s left shoulder) if the apex of the heart is pointing to the right of the patient’s body = dextrocardia, to the left = levocardia (normal!).
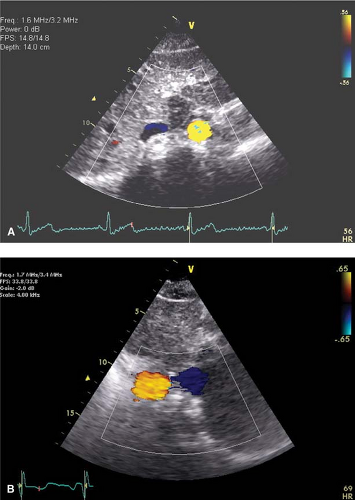
Establish situs of atria: Using a standard subcostal view of the aorta and IVC in cross section with color Doppler (probe perpendicular to spine with indicator pointing to the patient’s left hip), determine whether the aorta (red) is to the left and IVC (blue) to the right (situs solitus-normal!) or opposite (situs inversus) of the spine (Fig. 17-1). Atrial situs follows abdominal situs in ∼80% of patients.
Identify the morphologic RV: Trabeculated, moderator band present, apically displaced TV compared to MV, septal attachment of TV.
Establish the great vessels: PA early branching, aortic root gives off coronary arteries.
Key Point:
AV valves always stay with their respective ventricle, that is the apically displaced TV establishes its ventricle as the morphologic RV.
I. Simple shunts
KEY CONCEPTS
L → R shunt allows a portion of the pulmonary venous return to escape back to the lungs and reduces the cardiac output by the amount of the shunted volume.
R → L shunt: Oxygen content of systemic arterial blood falls in proportion to the volume of de-oxygenated systemic venous blood that has bypassed the lungs mixing with normal pulmonary venous return
To calculate shunt fraction (pulmonary flow/systemic flow = Qp/Qs) measure area of both LVOT and RVOT, trace PW Doppler through each valve to get VTI.
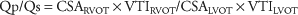
Qp/Qs >1: 1 indicates pulmonary flow exceed systemic flow and defines a net L → R shunt as volume of shunted oxygenated blood + venous blood entering the pulmonary circuit exceeds amount of blood leaving the systemic circuit.
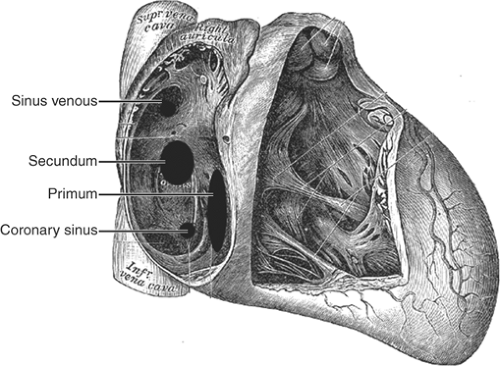
Figure 17-2. Anatomic distribution of ASD as seen from the right atrium. (Adapted from Gray, H. Anatomy of the Human Body, 20th ed. Philadelphia: Lea & Febiger, 1918.)
Qp/Qs <1: 1 indicates net R → L shunt as venous de-oxygenated blood mixes with oxygenated systemic blood.
Qp/Qs >1.5: 1 is considered a significant L → R shunt especially if right heart chamber dilatation is present.
A. ASD
See Figure 17-2.
Types:
Secundum: 75% of ASDs; located near the foramen ovale (mid interatrial septum).
Primum: 15% of ASDs; associated with AV canal defects (AVCD; base of interatrial septum).
Sinus venosus: 10% of ASDs; posterior edge of interatrial septum; usually associated with partial anomalous pulmonary venous return (typically right superior pulmonary vein).
Coronary sinus ASD: Rare; absence of the common wall that separates the LA from the coronary sinus as it courses in the AV groove to the RA. It is associated with persistent left superior vena cava.
Hemodynamics
Flow across an ASD is determined by the difference in compliance and capacity of both ventricles.
The thick walled LV is less compliant and under higher pressure than the thin walled RV and this favors L → R shunting.
Pulmonary blood flow is subsequently increased over time leading to RV volume overload (dilated RV, diastolic flattening of interventricular septum).
The right heart tolerates volume overload much better than pressure overload and therefore symptoms develop late, once pulmonary vascular resistance and pressures eventually increase.
Eisenmenger’s syndrome occurs when right heart pressures exceed left heart pressures promoting R → L shunt and hypoxemia.
Key Point:
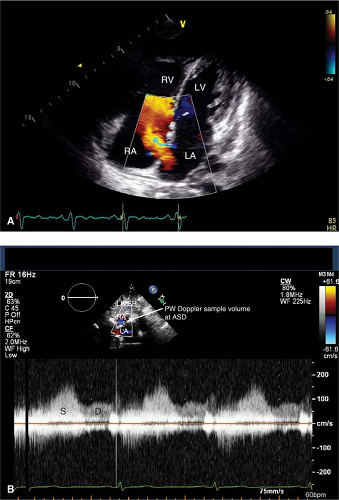
Best views to locate ASD are the PSAX, A4C, and subcostal. Beware of “drop-out” of the interatrial septum in the A4C view related to reduced lateral resolution of the ultrasound beam that may mimic a secundum ASD. This should be confirmed in the subcostal view where the interatrial septum is perpendicular to the ultrasound beam and also by color and pulsed-wave Doppler. Typically ASD flow begins in systole and continues throughout the cardiac cycle with a broad peak in late systole and early diastole (Fig. 17-3).
Key Point:
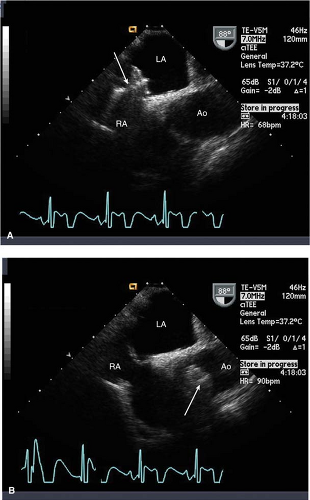
The subcostal and “off-axis” subcostal views are typically the only views in which a sinus venosus ASD may be visualized (Fig. 17-4). A TEE should be performed to assess for sinus venosus ASD in patients with unexplained right heart volume overload, especially if the agitated saline study is markedly positive without a shunt location identified.
Surgical repair
ASD closed if there is evidence of right ventricular dilatation or ASD diameter ≥10 mm
Surgical repair or trans-catheter closure
Echocardiography
TTE and TEE should document the type and size of ASD (ASD diameter) as well as direction (color and spectral Doppler), significance of shunt (QP/QS) and presence of associated congenital lesions.
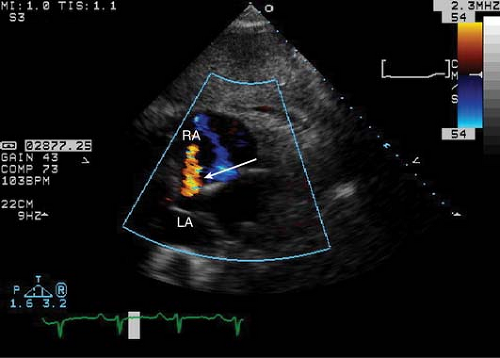
Post trans-catheter closure assess for maintenance of position of the occluder and any residual shunt (Fig. 17-5). Usually a small residual shunt is noted immediately after occluder placement, which should eventually cease.
Identify AV valve defects—for example, cleft MV in patients with ostium primum ASD.
Key Point:
Injection of agitated saline results in a quick (< 3 to 4 beats), intense opacification of the LV that clears with subsequent beats.
B. VSD
See Figure 17-6.
Types:
Perimembranous: 80% of VSDs, located near the septal leaflet of the TV and below the aortic valve.
Muscular: Either single or multiple (“Swiss-cheese” septum) involving the septum (Fig. 17-7).
Inlet: Involves the inflow portion of the septum and is associated with AVCD.
Supracristal or outflow tract: Involves RV outflow tract (above crista supraventricularis) and near the outflow valves; frequently occluded by a cusp of the aortic valve causing AR.
Hemodynamics
The majority of small single VSDs close spontaneously in childhood. A minority will persist into adulthood requiring surgical intervention.
The majority of flow occurs in systole resulting in an unmistakable, loud pansystolic murmur.
The shunt is initially L → R increasing flow to the pulmonary circulation and back to the LV.
Pulmonary artery systolic pressure (PASP) can be estimated, in the absence of pulmonic stenosis or RV outflow tract obstruction, if the systolic blood pressure (SBP) is known (PASP = SBP – 4VVSD2).
“Restrictive” VSDs are small high velocity/gradient jets (typically >75 mmHg) where there is rarely enough additional volume to cause an increase in pulmonary pressures or left heart dilatation (Fig. 17-8).
“Non-restrictive” VSDs are characterized by large defects with low peak velocity/gradient noted on spectral Doppler (typically <25 mmHg) suggesting that there is minimal pressure difference between LV and RV.
This results in a large transmission of volume to the pulmonary circulation and LV, leading to pulmonary vascular remodeling and an increase in resistance and pressure as well as left heart dilatation. Late in the natural history of this disease Eisenmenger’s s yndrome may occur.
Key Point:
Significant VSD shunt leads to LV, not RV, volume overload.
Surgical repair
Patch repair of VSD
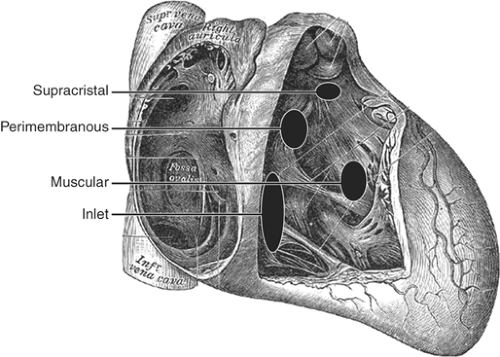 Figure 17-6. Anatomic distribution of VSD as seen from the right ventricle. (Adapted from Gray, H. Anatomy of the Human Body, 20th ed. Philadelphia: Lea & Febiger, 1918.) |
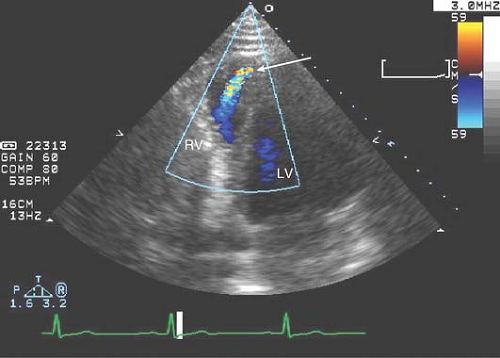 Figure 17-7. Off-axis A4C tilted toward the apex to reveal a congenital apical VSD with L → R flow (arrow) on color Doppler in a 23-year-old male with a loud systolic murmur. |
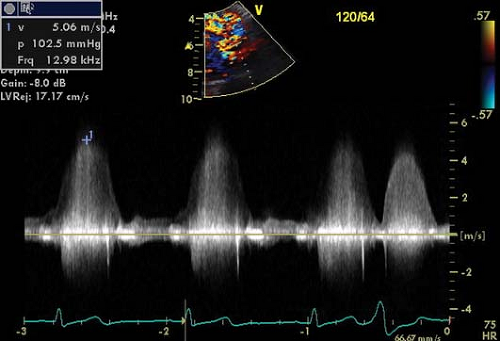 Figure 17-8. Systolic flow velocity seen on continuous-wave Doppler with a peak velocity (∼5 m/s) and gradient (∼103 mmHg) suggestive of a restrictive VSD.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|