TABLE 140.1 Approximate Frequencies of Congenital Heart Diseases, as Proportion of Total | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
TABLE 140.2 Syndromes and Congenital Heart Diseasea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 140.3 Recurrence Risk Siblings, Percent, After Diagnosis of Infant or Fetus with Congenital Heart Disease, by Type | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
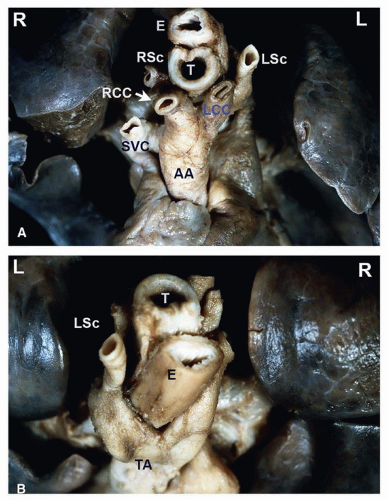
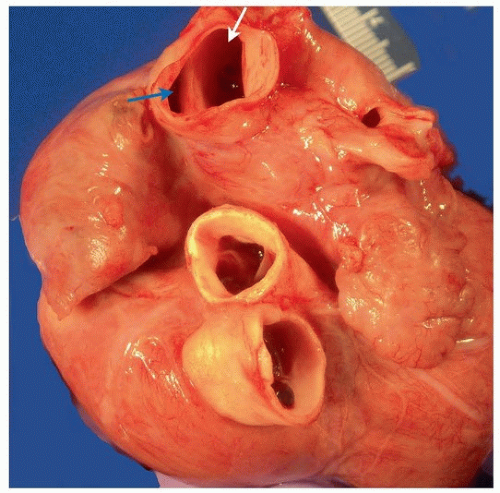
(both sides have typical right-sided features), and left isomerism (both sides have typical left-sided features). The spleen, being left sided, is absent in right-sided isomerism, on the right in situs inversus, and multiple in left-sided isomerism.
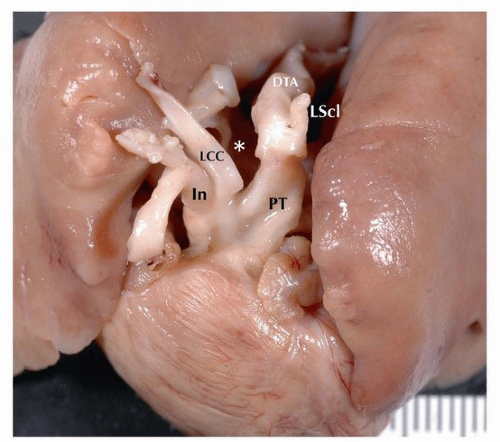
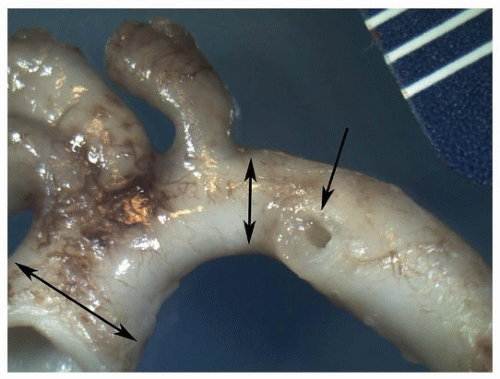 FIGURE 140.3 ▲ Normal infant aortic arch. Double arrows show ascending aorta (left) and isthmus (right). Single arrow shows the orifice of the ductus arteriosus. |
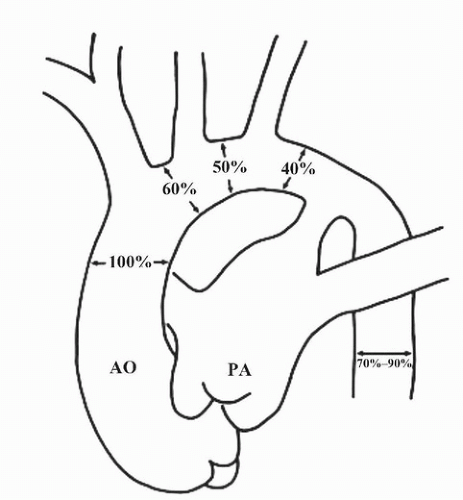
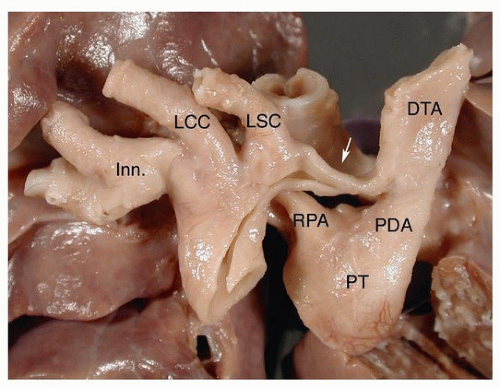
tetralogy, persistent truncus arteriosus, and pulmonary atresia and ventricular septal defect have right-sided arches with mirror-image branching (see below), in addition to lower rates for double outlet right ventricle, tricuspid atresia, and transposition.
that may range from sporadic migraine to transient ischemic attacks or stroke. Transthoracic echocardiography with contrast or transesophageal echocardiography is usually required to detect small PFO.
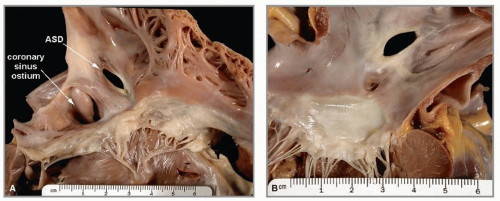
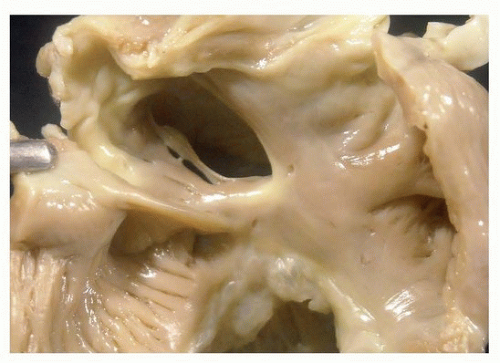 FIGURE 140.9 ▲ Atrial septal defect, secundum type, from the right. There is no septal tissue present in the oval fossa area, save a thin cord. The coronary sinus is present just below (posteriorly). |
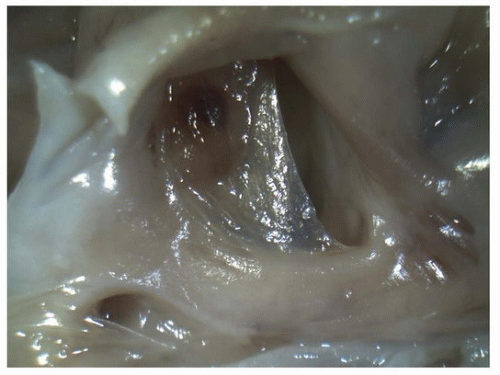
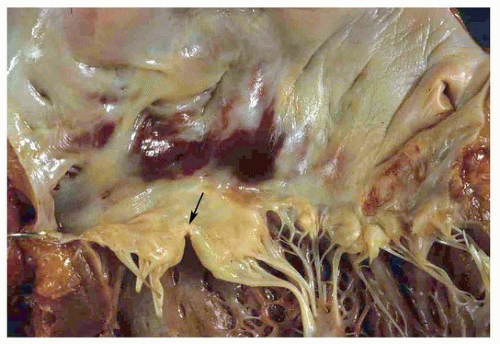 FIGURE 140.12 ▲ Partial atrioventricular canal defect. Note cleft of the anterior mitral valve leaflet (arrow). |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree