3 Congenital Heart Defects
I. LEFT-TO-RIGHT SHUNT LESIONS
A. ATRIAL SEPTAL DEFECT (OSTIUM SECUNDUM ASD)
PATHOLOGY AND PATHOPHYSIOLOGY
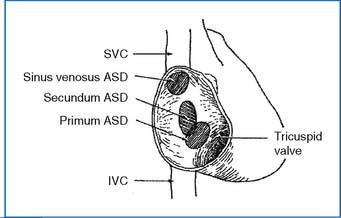
1. Three types of atrial septal defects (ASDs) occur in the atrial septum (Fig. 3-1). Secundum ASD is in the central portion of the septum and is the most common type (50% to 70% of ASDs). Primum ASD (or partial endocardial cushion defect [ECD]) is in the lower part of the septum (30% of ASDs). Sinus venosus defect is near the entrance of the superior vena cava (SVC) or inferior vena cava (IVC) to the right atrium (RA) (about 10% of all ASDs). Partial anomalous pulmonary venous return (PAPVR) is common with a sinus venous defect.
2. A left-to-right shunt (L-R shunt) is present through the defect, with a volume overload to the RA and right ventricle (RV) and an increase in pulmonary blood flow.
CLINICAL MANIFESTATIONS
1. The patients are usually asymptomatic.
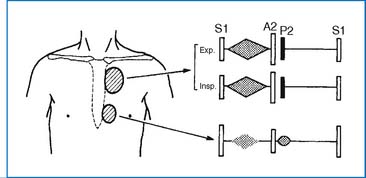
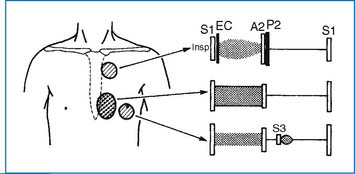
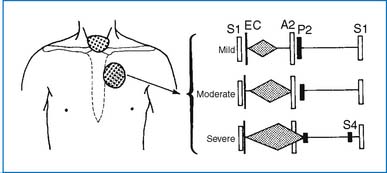
2. A widely split and fixed S2 and a grade 2 to 3/6 systolic ejection murmur at the upper left sternal border (ULSB) are characteristic of moderate-size ASD (Fig. 3-2). With a large L-R shunt a middiastolic rumble (resulting from relative tricuspid stenosis [TS]) may be audible at the lower left sternal border (LLSB). The typical auscultatory findings are usually absent in infants and toddlers, even in those with a large defect, because the RV is poorly compliant.
3. The ECG shows right axis deviation (RAD) (+90 to +180 degrees) and mild right ventricular hypertrophy (RVH) or right bundle branch block (RBBB) with an rsR′ pattern in V1.
4. Chest x-ray (CXR) films show cardiomegaly (with right atrial enlargement [RAE] and right ventricular enlargement [RVE]), increased pulmonary vascular markings (PVMs), and a prominent main pulmonary artery (MPA) segment.
5. Two-dimensional echo shows the position and the size of the defect. Cardiac catheterization is usually not necessary.
6. Spontaneous closure of the defect occurs more than 80% of the time in patients with defects 3 to 8 mm (diagnosed by echo) before 1½ years of age. An ASD with a diameter greater than 8 mm rarely closes spontaneously. The defect may reduce in size in some patients. If the defect is large and left untreated, pulmonary hypertension develops in the third and fourth decades of life. Cerebrovascular accident due to paradoxical embolization through an ASD is possible.
MANAGEMENT
MEDICAL
1. Exercise restriction is not required.
2. Nonsurgical closure of the defect using a catheter-delivered closure device has become a preferred method, provided the indications are met. These devices are applicable only to secundum ASD. The use of the closure device may be indicated for a defect measuring ≥5 mm in diameter (but <32 mm) with an adequate septal rim (4 mm), and evidence of RA and RV volume overload. Among several devices available, the Amplatzer Septal Occluder appears to be most popular. Following the device closure, the patients are placed on aspirin 81 mg per day for 6 months. Advantages of nonsurgical closure would include a hospital stay of less than 24 hours, rapid recovery, and no residual thoracotomy scar.
B. VENTRICULAR SEPTAL DEFECT
PATHOLOGY AND PATHOPHYSIOLOGY
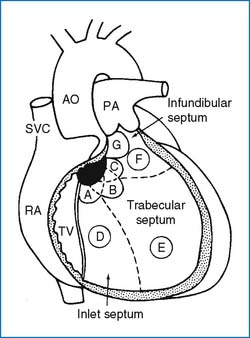
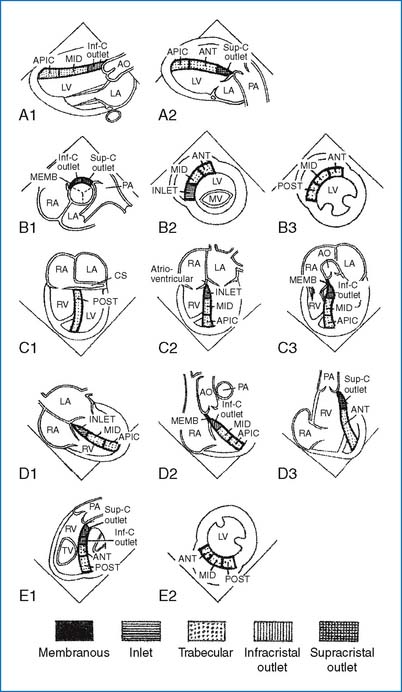
1. The ventricular septum consists of a small membranous septum and a larger muscular septum. The muscular septum has three components: the inlet, infundibular, and trabecular (or simply muscular) septa (Fig. 3-3). A membranous VSD often involves a varying amount of muscular septum adjacent to it (i.e., perimembranous VSD). The perimembranous defect is more common (70%) than the trabecular (5% to 20%), infundibular (5% to 7%), or inlet defects (5% to 8%). In Far Eastern countries the infundibular defects account for about 30%.
2. The perimembranous VSD is frequently associated with patent ductus arteriosus (PDA) and coarctation of aorta (COA). The VSD seen with tetralogy of Fallot (TOF) is a large nonrestrictive perimembranous defect with extension into the subpulmonary region. The inlet VSD is typically seen with endocardial cushion defects.
3. In subarterial infundibular or supracristal VSD the aortic valve may prolapse through the VSD, with resulting aortic regurgitation (AR) and reduction of the VSD shunt. The prolapse may occasionally occur with the perimembranous VSD.
4. In VSDs with small to moderate L-R shunts, volume overload is placed on the left atrium (LA) and left ventricle (LV) (but not on the RV). With larger defects the RV is also under volume and pressure overload, in addition to a greater volume overload on the LA and LV. Pulmonary blood flow (PBF) is increased to a varying degree depending on the size of the defect and the pulmonary vascular resistance. With a large VSD, pulmonary hypertension results. With a long-standing large VSD, pulmonary vascular obstructive disease (PVOD) develops, with severe pulmonary hypertension and cyanosis resulting from a right-to-left shunt (R-L shunt). At this stage, surgical correction is nearly impossible.
CLINICAL MANIFESTATIONS
1. Patients with small VSDs are asymptomatic, with normal growth and development. With large VSDs, delayed growth and development, repeated pulmonary infections, CHF, and decreased exercise tolerance are relatively common. With PVOD, cyanosis and a decreased level of activity may result.
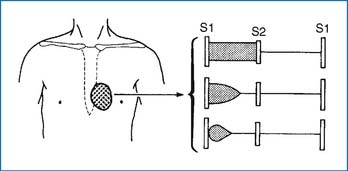
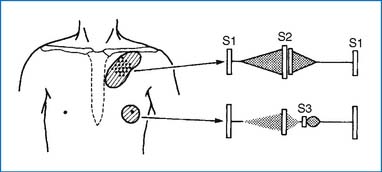
2. With a small VSD, a grade 2 to 5/6 regurgitant systolic murmur (holosystolic or less than holosystolic) maximally audible at the LLSB is characteristic (Fig. 3-4). A systolic thrill may be present at the LLSB. With a large defect, an apical diastolic rumble is audible, which represents a relative stenosis of the mitral valve due to large pulmonary venous return to the LA (Fig. 3-5). The S2 may split narrowly, and the intensity of the P2 increases if pulmonary hypertension is present (Fig. 3-5).
3. ECG findings: Small VSD, normal; moderate VSD, left ventricular hypertrophy (LVH) and left atrial hypertrophy (LAH) (±); large VSD, biventricular hypertrophy (BVH) and LAH (±); PVOD, pure RVH.
4. CXR films reveal cardiomegaly of varying degrees with enlargement of the LA, LV, and possibly the RV. PVMs are increased. The degree of cardiomegaly and the increase in PVMs are directly related to the magnitude of the L-R shunt. In PVOD the heart size is no longer enlarged and the MPA and the hilar pulmonary arteries are notably enlarged, but the peripheral lung fields are ischemic.
5. Two-dimensional echo studies provide accurate diagnosis of the position and size of the VSD. LA and LV dimensions provide indirect assessment of the magnitude of the shunt. Figure 3-6 shows selected 2D echo views of the ventricular septum, which helps locate the VSD position. The Doppler studies of the pulmonary artery (PA), tricuspid regurgitation (TR) (if present), and the VSD itself are useful in indirect assessment of RV and PA pressures (see Doppler Echocardiography, Chapter 2).
6. Spontaneous closure occurs in 30% to 40% of all VSDs, most often in small trabecular VSDs, more frequently in small defects than in large defects, and more often in the first year of life than thereafter. Large defects tend to become smaller with age. Inlet and infundibular VSDs do not become smaller or close spontaneously. CHF develops in infants with a large VSD but usually not until 6 or 8 weeks of age, when the PVR drops below a critical level. PVOD may begin to develop as early as 6 to 12 months of age in patients with a large VSD.
MANAGEMENT
MEDICAL.
Treatment of CHF with digitalis and diuretics (see Chapter 6). No exercise restriction is required in the absence of pulmonary hypertension.
SURGICAL
3. Surgical approaches for special situations
C. PATENT DUCTUS ARTERIOSUS
PATHOLOGY AND PATHOPHYSIOLOGY
1. There is a persistent postnatal patency of a normal fetal structure between the PA and the descending aorta.
2. The magnitude of the L-R shunt is determined by the diameter and length of the ductus and the level of PVR. With a long-standing large ductus, pulmonary hypertension and PVOD may develop with an eventual R-L shunt and cyanosis.
CLINICAL MANIFESTATIONS
1. Asymptomatic when the ductus is small. When the defect is large, signs of CHF may develop.
2. A grade 1 to 4/6 continuous (machinery) murmur best audible at the ULSB or left infraclavicular area is the hallmark of the condition (Fig. 3-7). An apical diastolic rumble is audible with a large-shunt PDA. Bounding peripheral pulses with wide pulse pressure are present with a large-shunt PDA.
3. ECG findings are similar to those of VSD: Normal or LVH in a small to moderate PDA; BVH in a large PDA; RVH if PVOD develops.
4. CXR findings are also similar to those of VSD: normal with a small-shunt PDA. With a large-shunt PDA, cardiomegaly (with LA and LV enlargement) and increased PVM are present. With PVOD the heart size is normal, with a marked prominence of the MPA and hilar vessels.
5. The PDA can be directly imaged and its hemodynamic significance determined by 2D echo and color flow Doppler examination. Cardiac catheterization is usually not indicated in isolated PDA.
6. CHF or recurrent pneumonia or both develop if the shunt is large. Spontaneous closure of PDA usually does not occur in term infants.
MANAGEMENT
MEDICAL
1. No exercise restriction is required in the absence of pulmonary hypertension.
2. Indomethacin is ineffective in term infants with PDA.
3. Catheter closure of the ductus may be employed. Small ductus <4 mm in diameter are closed by Gianturco stainless coils and larger ones by Amplatzer PDA device. An optimal candidate for the coil occlusion has the ductus 2.5 mm or less in size, but the use of multiple coils can close a ductus up to 5 mm. Amplatzer device may be used for PDAs ranging in size from 4 to 10 mm (with 100% closure rate). Complications may include residual leaks, pulmonary artery coil embolization, hemolysis, left PA stenosis, aortic occlusion with the Amplatzer device, and femoral vessel occlusion.
POSTSURGICAL FOLLOW-UP.
No restriction of activity is indicated unless pulmonary hypertension persists.
DIFFERENTIAL DIAGNOSIS.
1. Coronary atrioventricular (AV) fistula (the murmur is audible over the precordium, not at the ULSB)
2. Systemic AV fistula (a wide pulse pressure with bounding pulse, CHF, and a continuous murmur over the fistula [head or liver] are characteristic)
3. Pulmonary AV fistula (a continuous murmur over the back, cyanosis, and clubbing in the absence of cardiomegaly)
4. Venous hum (an innocent condition that disappears when the patient is supine)
5. Murmurs of collaterals in patients with COA or TOF (audible in the intercostal spaces)
6. VSD + AR (maximally audible at the mid-left sternal border [MLSB] or LLSB, it is actually a to-and-fro murmur, rather than a continuous murmur)
7. Absence of pulmonary valve (a to-and-fro murmur, or “sawing-wood sound,” at the ULSB; large central pulmonary arteries on CXR films; RVH on ECG; and cyanosis)
8. Persistent truncus arteriosus (occasional continuous murmur, cyanosis, BVH on the ECG, cardiomegaly, and increased PVM on CXR films)
9. Aortopulmonary septal defect (AP window) (bounding peripheral pulses, a murmur resembling that of VSD, and signs of CHF)
10. Peripheral PA stenosis (a continuous murmur may be audible all over the thorax, unilateral or bilateral)
11. Ruptured sinus of Valsalva aneurysm (sudden onset of chest pain and severe heart failure, a continuous murmur or a to-and-fro murmur, and often Marfan features)
12. Total anomalous pulmonary venous return (TAPVR) draining into the RA (a murmur similar to venous hum along the right sternal border, mild cyanosis, RVH on ECG, and cardiomegaly with increased PVM on CXR)
D. PATENT DUCTUS ARTERIOSUS IN PRETERM NEONATES
PATHOPHYSIOLOGY
1. PDA is a special problem in premature infants with hyaline membrane disease. With improvement in oxygenation the PVR falls rapidly, but the ductus remains patent because its responsiveness to oxygen is immature in premature newborns. The resulting large L-R shunt makes the lung stiff, and weaning the infant from the ventilator and oxygen therapy becomes difficult.
2. If the ductus is not closed, the infant remains on ventilator therapy, with development of bronchopulmonary dysplasia and pulmonary hypertension (cor pulmonale) with right-sided heart failure.
CLINICAL MANIFESTATIONS
1. It is important to predict a significant PDA in a premature neonate in whom weaning from ventilator is delayed or fails. Episodes of apnea or bradycardia may be the initial sign of PDA in infants who are not on ventilators.
2. The physical examination reveals bounding peripheral pulses, a hyperactive precordium, and tachycardia with or without gallop rhythm. The classic continuous murmur at the left infraclavicular area or ULSB is diagnostic, but the murmur may be only systolic and is difficult to hear in infants who are on ventilators.
3. The ECG is usually normal but occasionally shows LVH.
4. CXR films show cardiomegaly and increased PVM in larger premature infants who are not intubated. In infants who are intubated and on high ventilator settings, CXR films may show the heart to be either of normal size or only mildly enlarged.
5. Two-dimensional echo and color flow Doppler studies provide accurate anatomic and functional information. Doppler studies of the ductus (with the sample volume placed at the pulmonary end of the ductus) provide important functional information, such as ductal shunt patterns (pure left-to-right, bidirectional, or predominant R-L shunt), pressures in the PA, and magnitude of the ductal shunt or pulmonary perfusion status.
MEDICAL
1. Fluid restriction to 120 mL/kg per day and a diuretic (e.g., furosemide, 1 mg/kg, 2 to 3 times a day) may be tried for 24 to 48 hours, but these regimens have a low success rate. Digoxin is not used because it has little hemodynamic benefit and a high incidence of digitalis toxicity.
2. Pharmacologic closure of the PDA can be achieved with intravenous administration of indomethacin, every 12 hours, a total of three doses. One example of the dosage regimens is as follows. The dose is given intravenously every 12 hours, a total of three doses. For patients younger than 48 hours old, 0.2 mg/kg is followed by 0.1 mg/kg times 2; for patients 2 to 7 days old, 0.2 mg/kg times 3; and for those older than 7 days old, 0.2 mg/kg followed by 0.25 mg/kg times 2. A second course of indomethacin treatment is occasionally necessary to achieve adequate ductal closure. Contraindications to the use of indomethacin include high blood urea nitrogen (>25 mg/dL) or creatinine (>1.8 mg/dL) levels, low platelet count (<80,000/mm3), bleeding tendency (including intracranial hemorrhage), necrotizing enterocolitis, and hyperbilirubinemia.
3. In Europe, intravenous ibuprofen (10 mg/kg, followed at 24-hour intervals by two doses of 5 mg/kg) is popular. Ibuprofen appears to have a significantly lower incidence of oliguria and a less deleterious effect on cerebral blood flow.
E. COMPLETE ENDOCARDIAL CUSHION DEFECT (COMPLETE AV CANAL)
PATHOLOGY AND PATHOPHYSIOLOGY
1. Complete ECD consists of an ostium primum ASD, an inlet VSD, and clefts in the anterior mitral valve leaflet and in the septal leaflet of the tricuspid valve, forming common anterior and posterior cusps of the AV valve (Fig. 3-8). When the ventricular septum is intact, the defect is termed partial ECD or ostium primum ASD.
2. In complete ECD a single valve orifice connects the atrial and ventricular chambers, whereas in the partial form there are separate mitral and tricuspid orifices. In the majority of complete ECDs the AV valve orifice is equally committed to the RV and LV. In some patients, however, the orifice is committed primarily to one ventricle, with hypoplasia of the other ventricle (i.e., “unbalanced” AV canal with RV or LV dominance). Hypoplasia of one ventricle may necessitate one ventricular repair (Fontan-type operation).
3. Additional cardiac anomalies may include TOF (called “canal tet,” occurring in 6% of patients with ECD), double-outlet right ventricle (DORV) with more than 50% overriding of the aorta (occurring in 6%), and transposition of the great arteries (TGA) (occurring in 3%). Associated defects are rare in children with Down syndrome.
4. The combination of these defects may result in an interatrial and/or interventricular shunt, AV valve regurgitation, or LV-to-RA shunt. CHF with or without pulmonary hypertension usually develops early in infancy.
CLINICAL MANIFESTATIONS
1. Failure to thrive, repeated respiratory infections, and signs of CHF are common during early infancy.
2. Hyperactive precordium with a systolic thrill at the LLSB and a loud S2 are frequent findings. A grade 3 to 4/6 holosystolic regurgitant murmur is audible along the LLSB. The systolic murmur of mitral regurgitation (MR) may be audible at the apex. A middiastolic rumble at the LLSB or at the apex (from relative stenosis of the tricuspid and/or mitral valve) and gallop rhythm may be present.
3. The ECG finding of a “superior” QRS axis (with the axis between −40 and −150 degrees) is characteristic. RVH or RBBB is present in all, and many have LVH as well. Most patients have a prolonged PR interval (first-degree AV block).
4. CXR films always show cardiomegaly with increased PVMs.
5. Two-dimensional echo and color flow Doppler studies allow imaging of all components of ECD, as well as an assessment of the hemodynamic severity.
6. CHF occurs 1 to 2 months after birth, and recurrent pneumonia is commonly seen. Children with Down syndrome and ECD begin to develop PVOD in infancy. The survivors develop PVOD and die in late childhood or as young adults.
MANAGEMENT
SURGICAL
1. PA banding is no longer recommended unless other associated anomalies make complete repair a high-risk procedure. The mortality rate for PA banding is as high as 15%.
2. Closure of ASD and VSD and reconstruction of cleft AV valves under cardiopulmonary bypass and/or deep hypothermia are carried out between 2 and 4 months of age. Surgical mortality is 3% to 10%. Most of these infants have CHF that is unresponsive to medical therapy, and some have elevated PVR. Early surgical repair of the defect is especially important for infants with Down syndrome because of their known tendency to develop early PVOD.
3. Complications of the surgery include MR (which is persistent or has been worsened), sinus node dysfunction (with resulting bradyarrhythmias), and complete heart block (occurring in <5% of the patients).
4. Those patients with unbalanced AV canal (with hypoplasia of right or left ventricle) may be treated by an earlier PA banding and later by a modified Fontan operation.
5. In patients with “canal tet” who are severely cyanotic a systemic-to-PA shunt is carried out during infancy and a complete repair done between 2 and 4 years of age.
F. PARTIAL ENDOCARDIAL CUSHION DEFECT (OSTIUM PRIMUM ASD)
PATHOLOGY AND PATHOPHYSIOLOGY
1. A defect is present in the lower part of the atrium septum near the AV valves, without an interventricular communication (Fig. 3-8). The anterior and posterior bridging leaflets are fused by a connecting tongue to form separate right and left AV orifices. Clefts of the mitral and occasionally of the tricuspid valve are present.
2. Less common forms of partial ECD include common atrium (which is a characteristic lesion seen in Ellis-van Creveld syndrome), VSD of the inlet septum (i.e., AV canal-type VSD), and isolated cleft of the mitral valve.
3. Pathophysiology of ostium primum ASD is similar to that of ostium secundum ASD.
CLINICAL MANIFESTATIONS
1. Usually asymptomatic during childhood.
2. Physical findings are identical to those of secundum ASD (Fig. 3-2), except for a regurgitant systolic murmur of MR, which may be present at the apex.
3. The ECG shows a “superior” QRS axis, as in complete ECD. First-degree AV block (50%) and RVH or RBBB (rsR′ pattern in V1) are common.
4. CXR findings are identical to those of secundum ASD except for the enlargement of the LA and LV when MR is significant.
5. Two-dimensional echo allows accurate diagnosis of primum ASD.
6. CHF may develop in childhood and pulmonary hypertension in adulthood. Spontaneous closure of the defect does not occur. Cardiac arrhythmias (20%) may complicate the defect.
G. PARTIAL ANOMALOUS PULMONARY VENOUS RETURN
PATHOLOGY AND PATHOPHYSIOLOGY
1. One or more but not all pulmonary veins (PVs) drain into the RA or its venous tributaries such as the SVC, IVC, coronary sinus, or left innominate vein.
2. The right PVs are involved twice as often as the left PVs. The right PVs may drain into the SVC, often associated with sinus venous ASD, or drain into the IVC in association with an intact atrial septum and bronchopulmonary sequestration. The left PVs drain either into the left innominate vein or into the coronary sinus.
3. The hemodynamic alteration is similar to that seen with ASD. The magnitude of the pulmonary blood flow is determined by the number of anomalous PVs and the presence and size of the ASD.
CLINICAL MANIFESTATIONS
1. Children with PAPVR are usually asymptomatic.
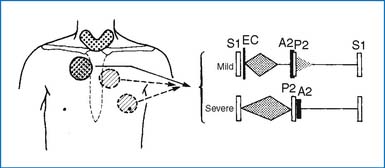
2. Physical findings are similar to those of ASD (Fig. 3-2). When associated with ASD, the S2 is split widely and fixed. When the atrial septum is intact, the S2 is normal.
3. The ECG shows RVH or RBBB or is normal.
4. CXR films show RAE, RVE, and increased PVMs.
5. Echo diagnosis of PAPVR is less reliable.
6. If PAPVR is undetected, cyanosis and exertional dyspnea may develop during the third and fourth decades, resulting from pulmonary hypertension and PVOD.
MANAGEMENT
SURGICAL
1. Surgical correction is carried out when the patient is 2 to 5 years of age. A significant L-R shunt with Qp/Qs greater than 1.5:1 or 2:1 is an indication for surgery. Isolated single lobe anomaly is not ordinarily corrected.
2. For the anomalous drainage into the SVC a tunnel is created between the anomalous vein and the ASD using a Teflon or pericardial patch and the SVC is widened to prevent obstruction of flow. For the anomalous drainage into the IVC an intraatrial tunnel drains the venous blood into the LA. When this is associated with the bronchopulmonary sequestration, the involved lobes(s) may be resected (without connecting the anomalous vein to the heart). When the left PVs drain into the coronary sinus, the sinus is unroofed and the orifice of the coronary sinus is closed.
II. OBSTRUCTIVE LESIONS
A. PULMONARY STENOSIS
PATHOLOGY AND PATHOPHYSIOLOGY
1. Pulmonary stenosis (PS) may be valvular (90%), subvalvular (infundibular), or supravalvular (i.e., stenosis of the PA). In valvular PS the pulmonary valve is thickened, with fused or absent commissures and a small orifice. A poststenotic dilation of the MPA usually develops in valvular PS. Dysplastic pulmonary valve (with thickened, irregular, immobile tissue) is frequently seen with Noonan syndrome. Infundibular PS is usually associated with a large VSD, as seen in TOF. The poststenotic dilation is not seen with subvalvular stenosis.
2. Abnormal muscular bands (running between the ventricular septum and the anterior wall) divide the RV cavity into a proximal high-pressure chamber and a distal low-pressure chamber (called “double-chambered” RV).
3. Depending on the severity of PS, a varying degree of RVH develops. The RV is usually normal in size but, with critical pulmonary stenosis, the RV is hypoplastic.
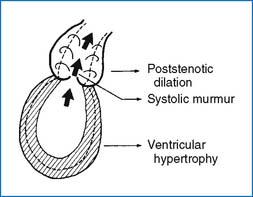
4. In general, three pathophysiologic changes occur in obstructive lesions such as PS or aortic stenosis (AS). They are (1) systolic ejection murmur on auscultation, (2) hypertrophy of the responsible ventricle, and (3) poststenotic dilation (Fig. 3-9).
CLINICAL MANIFESTATIONS
1. Usually asymptomatic with mild PS. Exertional dyspnea and easy fatigability may be seen in moderately severe cases and CHF occurs in severe cases. Neonates with critical PS are cyanotic and tachypneic.
2. An ejection click is present at the ULSB with valvular PS (Fig. 3-10). The S2 may split widely, and the P2 may be diminished in intensity. A systolic ejection murmur (grade 2 to 5/6) with or without systolic thrill is best audible at the ULSB and transmits fairly well to the back and the sides. The louder and longer the murmur, the more severe is the stenosis. Neonates with critical PS may have only a faint heart murmur, if any.
3. The ECG is normal in mild PS. RAD and RVH are present in moderate PS. RAH and RVH with “strain” pattern are present in severe PS. Neonates with critical PS may show LVH (due to hypoplastic RV and relatively large LV).
4. CXR films show normal heart size and a prominent MPA segment (i.e., poststenotic dilation). PVMs are normal but may be decreased in severe PS.
5. Two-dimensional echo may show thick pulmonary valves with restricted systolic motion (doming) and a poststenotic dilation of the MPA. The Doppler study can estimate the pressure gradient across the stenotic valve.
6. The severity of the obstruction is usually not progressive in mild PS, but it tends to progress with age in moderate or severe PS. CHF may develop in patients with severe stenosis. Sudden death during heavy physical activities is possible in patients with severe stenosis.
MANAGEMENT
MEDICAL
1. For neonates with critical PS and cyanosis, prostaglandin E1 (PGE1) infusion to reopen the ductus should be started. Balloon valvuloplasty is the procedure of choice in critically ill neonates. Even dysplastic valves appear to mature after the procedure. Some patients require reintervention (either repeat valvuloplasty or surgery) at a later time.
2. Balloon valvuloplasty is the procedure of choice for significant pulmonary valve stenosis. Indications for the balloon procedure include (a) symptomatic patients (with angina, syncope or presyncope, and exertional dyspnea) with catheterization pressure gradient ≥30 mm Hg and (b) asymptomatic patients with cath gradient greater than 40 mm Hg. Cardiac catheterization is recommended in patients with a Doppler-estimated pressure gradient near 50 mm Hg. This procedure is useful even for dysplastic pulmonary valve.
3. Surgical treatment is not possible for PA branch stenosis that is within the lung parenchyma. In such cases the balloon angioplasty with balloon-expandable intravascular stent is effective (with effectiveness of 75% to 100%).
4. Restriction of activity is usually not indicated except for severe PS.
SURGICAL
1. Surgical valvotomy is occasionally indicated in patients with valvular PS in whom balloon valvuloplasty is unsuccessful.
2. Surgery is indicated in patients with dysplastic pulmonary valves that are resistant to dilation. Dysplastic valve may need to be completely excised because simple valvotomy may be ineffective.
3. Surgery is also indicated for infundibular stenosis and anomalous RV muscle bundle with significant pressure gradients.
4. If balloon valvuloplasty is unsuccessful, infants with critical PS require surgery on an urgent basis.
5. Stenosis at the main PA requires patch widening of the narrow portion.
B. AORTIC STENOSIS
PATHOLOGY AND PATHOPHYSIOLOGY
1. Left ventricular outflow tract (LVOT) obstruction may occur at the valvular, subvalvular, or supravalvular levels (Fig. 3-11).
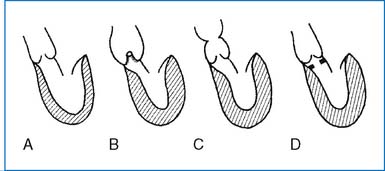
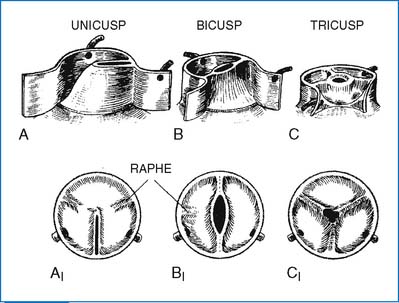
2. Valvular AS is caused most often by a bicuspid aortic valve (with a fused commissure) and less commonly by a unicuspid valve (with one lateral attachment) or stenosis of the tricuspid valve (Fig. 3-12). Many cases of bicuspid aortic valve are nonobstructive during childhood.
3. Symptomatic neonates with so-called critical neonatal aortic valve stenosis have primitive, myxomatous valve tissue, with a pinhole opening. The aortic valve ring and ascending aorta, the mitral valve, and the LV cavity are almost always hypoplastic (often requiring a Norwood operation followed by a Fontan operation).
4. Supravalvular AS occurs at the upper margin of the sinus of Valsalva. This is often associated with Williams syndrome.
5. Subvalvular (subaortic) stenosis may be in the form of simple diaphragm (discrete) or a long, tunnel-like fibromuscular narrowing (tunnel stenosis).
6. Hypertrophy of the LV may develop if the stenosis is severe. A poststenotic dilation of the ascending aorta develops with valvular AS. AR usually develops in subaortic AS.
CLINICAL MANIFESTATIONS
1. Patients with mild to moderate AS are asymptomatic. Exertional chest pain or syncope may occur with severe AS. CHF develops within the first few months of life with critical AS.
2. In symptomatic infants with critical AS the heart murmur may be absent or faint, and the peripheral pulses are weak and thready.
3. Blood pressure is normal in most patients, but a narrow pulse pressure is present in severe AS. Patients with supravalvular AS may have a higher systolic pressure in the right arm than in the left (due to the jet of stenosis directed into the innominate artery, the so-called Coanda effect).
4. A systolic thrill may be present at the upper right sternal border (URSB), in the suprasternal notch, or over the carotid arteries. An ejection click may be audible with valvular AS. A harsh systolic ejection murmur (grade 2 to 4/6) is best audible at the second right intercostal space (2RICS) or third left intercostal space (3LICS) (Fig. 3-13), with good transmission to the neck and frequently to the apex. A high-pitched, early diastolic decrescendo murmur of AR may be audible in patients with bicuspid aortic valve and those with discrete subvalvular stenosis.
5. The ECG is normal in mild cases. LVH with or without a strain pattern is seen in more severe cases.
6. CXR films are usually normal in children, but a dilated ascending aorta may be seen occasionally in valvular AS. A significant cardiomegaly develops with CHF or substantial AR.
7. Echo studies are diagnostic. Two-dimensional echo may show the anatomy of the aortic valve (bicuspid, tricuspid, or unicuspid) and that of subvalvular and supravalvular AS. The Doppler-derived pressure gradient (instantaneous gradient) is approximately 20% higher than the peak-to-peak systolic pressure gradient obtained during cardiac catheterization. The degree of LV hypertrophy can be measured.
8. Mild stenosis becomes frequently more severe with time. The stenosis may worsen with aging as the result of calcification of the valve cusps (requiring valve replacement surgery in some adult patients). Progressive AR is possible in discrete subaortic stenosis.
MANAGEMENT
MEDICAL
1. In critically ill neonates and infants with CHF, anticongestive measures with fast-acting inotropic agents and diuretics, with or without PGE1 infusion, are indicated, in preparation for either balloon valvuloplasty or surgery.
2. Serial echo-Doppler studies are needed every 1 to 2 years because AS of all severities tends to worsen with time. Exercise stress test (EST) may be indicated in asymptomatic children who want to participate in sports activities.
3. Percutaneous balloon valvuloplasty is now regarded as the first step in management of symptomatic neonates in many centers. It is also the first interventional method for children older than 1 year of age. Although the results are promising, they are not as good as those for PS. A survival rate of 50% has been reported in neonates. Serious complications (major hemorrhage, loss of femoral artery pulse, avulsion of part of the aortic valve leaflet, perforation of the mitral valve or LV) can occur. Indications for the balloon procedure are as follows:
4. Activity restrictions. No limitation in activity is required for mild AS (with peak Doppler gradient <40 mm Hg). Moderate AS (peak Doppler gradient 40 to 70 mm Hg) requires restriction from high dynamic or static competitive athletics (allowing only golf, baseball, doubles tennis, etc.). With severe AS (peak Doppler gradient >70 mm Hg), no competitive sports are allowed.
SURGICAL.
Generally accepted indications for surgery and surgical procedures are as follows:
1. For valvular AS, failed balloon valvuloplasty or severe AR resulting from the procedure is an indication. A sick newborn with critical AS who has failed balloon valvuloplasty requires surgery. Surgery is indicated in children with symptoms (chest pain, syncope) with a strain pattern on the ECG or abnormal exercise test, even with a systolic pressure gradient slightly less than 50 mm Hg.
2. For discrete subaortic stenosis, a systolic pressure gradient greater than 30 mm Hg or the onset of an AR is an indication for an elective operation. Some centers consider the mere presence of a significant membrane as an indication for surgery. Excision of the discrete membrane is performed. It is advisable to wait if possible until beyond 10 years of age because the recurrence rate of the subaortic membrane is higher before that age.
3. For tunnel-type subaortic stenosis, a pressure gradient ≥50 mm Hg is an indication. Valve replacement following aortic root enlargement (Kono procedure) may be performed.
4. For supravalvular AS, the peak pressure gradient greater than 50 to 60 mm Hg, severe LVH, or appearance of new AR is an indication for surgery. Widening of the stenotic area using a diamond-shaped fabric patch may be performed.
POSTSURGICAL OR POSTPROCEDURAL FOLLOW-UP
1. Annual follow-up is required for all patients who have had a balloon or surgical procedure done for the aortic valve because significant AR develops in 10% to 30% of the patients and the discrete subaortic membrane recurs in 25% to 30% after surgical resection.
2. Anticoagulation is needed after a prosthetic mechanical valve replacement. The INR should be maintained between 2.5 and 3.5 for the first 3 months and 2.0 to 3.0 beyond that time. Low-dose aspirin (81 mg per day) is also indicated in addition to warfarin.
3. After aortic valve replacement with bioprosthesis, aspirin (81 mg) is indicated (without warfarin).
4. Subacute bacterial endocarditis (SBE) prophylaxis is required after placement of prosthetic material or valve, when indications arise.
5. Restriction from competitive sports may be necessary for children with moderate residual AS and/or AR.
C. COARCTATION OF THE AORTA
PATHOLOGY AND PATHOPHYSIOLOGY
1. In COA a narrowing of the upper thoracic aorta is present. There are two groups of patients with COA: one group of patients presenting symptoms early in life and the other group remaining asymptomatic.
2. COA also occurs as part of other CHDs, such as TGA and DORV (e.g., Taussig-Bing abnormality).
3. As many as 85% of patients with COA have a bicuspid aortic valve.
CLINICAL MANIFESTATIONS
1. Signs of CHF (poor feeding, dyspnea) and renal failure (oliguria, anuria) with general circulatory shock may develop in the first 2 to 6 weeks of life.
2. A loud gallop is usually present, but heart murmur may be absent with weak and thready pulses in sick infants.
3. The ECG usually shows RVH or RBBB rather than LVH.
4. CXR films show a marked cardiomegaly and signs of pulmonary edema or pulmonary venous congestion.
5. Two-dimensional echo shows the site and extent of the COA and other associated cardiac defects. The Doppler examination reveals a disturbed flow distal to the COA and signs of delayed emptying in the proximal aorta.
6. In symptomatic infants with COA, early death from CHF and renal failure is possible.
MANAGEMENT
MEDICAL
1. Intensive anticongestive measures should be given with fast-acting inotropic agents (catechols), diuretics, and oxygen to stabilize the patient. PGE1 infusion is indicated to reopen the ductus before any intervention takes place (see Appendix E for the dosage of PGE1).
2. Balloon angioplasty is controversial, but it can be a useful procedure for sick infants. However, balloon angioplasty is associated with a higher rate of recoarctation (>50%) than surgical repair, and the rate of complications (including femoral artery injury) is high during infancy.
SURGICAL
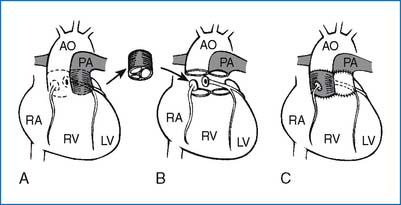
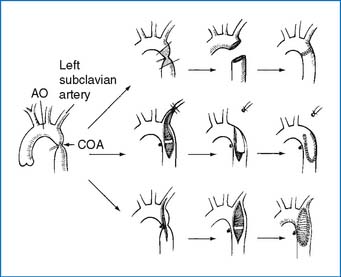
1. If CHF develops, the need for surgery or nonsurgical intervention is urgent. Surgical procedure of choice varies from institution to institution. Resection and end-to-end anastomosis (preferable when possible), subclavian flap aortoplasty, or patch angioplasty is performed (Fig. 3-15). The mortality rate for isolated COA is less than 5%. Postoperative renal failure is the most common cause of death. Residual obstruction and/or recoarctation occur in up to one third of all cases, but the recurrence rate is lower than that following balloon angioplasty.
2. If it is associated with a VSD, one of the following procedures may be performed.
Asymptomatic Children
CLINICAL MANIFESTATIONS
1. These patients are usually asymptomatic except for rare complaints of leg pain.
2. The pulse in the leg is absent or weak and delayed. Hypertension in the arm or higher blood pressure (BP) readings in the arm than the thigh may be present. An ejection click resulting from the bicuspid aortic valve is frequently audible at the apex and/or base. A systolic ejection murmur, grade 2 to 3/6, is audible at the URSB and MLSB and in the left interscapular area in the back.
3. The ECG usually shows LVH, but it may be normal.
4. CXR films show a normal or slightly enlarged heart. An E sign on the barium-filled esophagus or 3 sign on overpenetrated films may be found. Rib notching may be seen in children after about 5 years of age.
5. Two-dimensional echo shows a discrete, shelflike membrane in the posterolateral aspect of the descending aorta. The Doppler examination reveals disturbed flow and increased flow velocity distal to the coarctation. The full Bernoulli equation (using the flow velocities proximal and distal to the coarctation site) provides more accurate assessment of the severity of the obstruction. The bicuspid aortic valve is frequently imaged.
6. The presence of PDA makes the diagnosis of COA less certain in neonates. In addition to the presence of a posterior shelf and BP discrepancies between the arms and the legs, the ratio of isthmus to descending aorta diameter (measured at the level of diaphragm) less than 0.64 strongly suggests COA in the presence of PDA (Lu et al. J Pediatr. 2006). Others suggest that the isthmic diameter ≤3 mm or the isthmic diameter 4 mm plus the continuous antegrade flow by Doppler is a probable sign of PDA + COA.
7. Bicuspid aortic valve may cause stenosis and/or regurgitation later in life. If a COA is left untreated, LV failure, intracranial hemorrhage, or hypertensive encephalopathy may develop in childhood or adult life.
MANAGEMENT
MEDICAL
1. Hypertension or hypertensive crisis should be detected and treated.
2. Balloon angioplasty for native (unoperated) COA is controversial. Some centers use the balloon procedure for native COA, while other centers prefer a surgical approach. There appears to be a higher incidence of aortic aneurysm formation following the balloon procedure than surgery.
3. A balloon-expandable, stainless-steel stent implanted concurrently with balloon angioplasty is in the early stage of experience. An absorbable metal stent is also in the experimental stage.
SURGICAL
2. Resection of the coarctation segment and end-to-end anastomosis constitute the procedure of choice. Other surgical options are illustrated in Figure 3-15.
POSTOPERATIVE
1. Annual examination should be performed with attention to (a) BP differences in the arms and leg (recoarctation), (b) persistence or resurgence of hypertension in the arms and legs, (c) status of associated abnormalities such as bicuspid aortic valve or mitral valve disease, and (d) possible development of subaortic AS.
2. Residual pressure gradient is common but usually less than 10 to 20 mm Hg.
3. If COA recurs following either surgery or angioplasty, balloon angioplasty is the procedure of choice.
D. INTERRUPTED AORTIC ARCH
PATHOLOGY AND PATHOPHYSIOLOGY
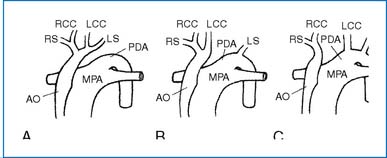
1. This extreme form of COA is divided into three types according to the location of the interruption (Fig. 3-16).
2. PDA and VSD are almost always associated with this defect. A bicuspid aortic valve (60%), mitral valve deformity (10%), persistent truncus arteriosus (10%), or subaortic stenosis (20%) may be present.
3. DiGeorge syndrome occurs in at least 15% of these patients.
CLINICAL MANIFESTATIONS
1. Respiratory distress, cyanosis, poor peripheral pulse, or circulatory shock develops in the first few days of life.
2. Cardiac findings are nonspecific.
3. CXR films show cardiomegaly, increased PVMs, and pulmonary edema. The upper mediastinum may be narrow (due to the absence of thymus, i.e., DiGeorge syndrome).
5. Echo studies are useful in the diagnosis of the condition. Angiocardiography is usually indicated for accurate diagnosis of the anatomy before surgery.
MANAGEMENT
1. Medical treatment consists of PGE1 infusion (see Appendix E for dosage), intubation, and oxygen administration. Workup for DiGeorge syndrome (i.e., serum calcium) should be carried out. Citrated blood (that causes hypocalcemia by chelation) should not be transfused, and blood should be irradiated before transfusion in patients with DiGeorge syndrome.
2. Surgical repair of the interruption (primary anastomosis, Dacron vascular graft, or venous homograft) and closure of a simple VSD are recommended if possible. If the interruption is associated with complex defects, repair of the interruption and PA banding are performed, with complete repair later.
III. CYANOTIC CONGENITAL HEART DEFECTS
A. COMPLETE TRANSPOSITION OF THE GREAT ARTERIES
PATHOLOGY AND PATHOPHYSIOLOGY
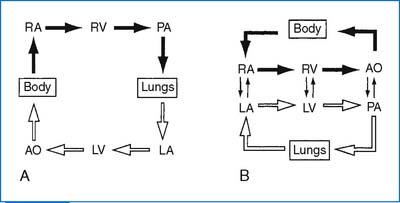
1. The aorta (AO) and the pulmonary artery are transposed, with the AO arising anteriorly from the RV, and the PA arising posteriorly from the LV. The end result is complete separation of the two circuits, with hypoxemic blood circulating in the body and hyperoxemic blood circulating in the pulmonary circuit (Fig. 3-17). The classic complete TGA is called D-transposition, in which the aorta is located anteriorly and to the right of the PA, hence D-TGA. (When the transposed aorta lies to the left of the PA, it is called L-transposition.)
2. Defects that permit mixing of the two circulations, such as ASD, VSD, and PDA, are necessary for survival. A VSD is present in 40% of cases. In about 50% of the patients no associated defects are present other than patent foramen ovale (PFO), small ASD, or small PDA.
3. LVOT obstruction (subpulmonary stenosis) occurs in about 5% of the patients without VSD, either dynamic or fixed obstruction. PS occurs in 30% to 35% of patients with VSD.
4. In neonates with poor mixing of the two circulations, progressive hypoxia and acidosis result in early death, requiring an early intervention.
CLINICAL MANIFESTATIONS
1. Cyanosis and signs of CHF develop in the newborn period.
2. Severe arterial hypoxemia unresponsive to oxygen inhalation and acidosis are present in infants with poor mixing. Hypoglycemia and hypocalcemia are occasionally present.
3. Auscultatory findings are nonspecific. The S2 is single and loud. No heart murmur is audible in infants with an intact ventricular septum. When TGA is associated with VSD or PS, a systolic murmur of these defects may be audible.
4. The ECG shows RAD and RVH. An upright T wave in V1 after 3 days of age may be the only abnormality suggestive of RVH. BVH may be present in infants with large VSD, PDA, or PS.
5. CXR films show cardiomegaly with increased PVMs. An egg-shaped cardiac silhouette with a narrow superior mediastinum is characteristic.
6. Two-dimensional echo study is diagnostic. It fails to show a “circle-and-sausage” pattern of the normal great arteries in the parasternal short-axis view. Instead, it shows two circular structures. Other views reveal the PA arising from the LV and the aorta arising from the RV. Associated anomalies (VSD, LVOT obstruction, PS, ASD, and PDA) are imaged.
7. Natural history and prognosis depend on anatomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree