1. Anomalous origin of a coronary artery from the opposite sinus of Valsalva
(a) Anomalous origin of the left coronary from the right sinus
(b) Anomalous origin of the right coronary from the left sinus
2. Anomalous origin of the left coronary artery from the pulmonary artery
3. Coronary artery fistulae
Role and Utility of TEE in the Evaluation of Congenital Coronary Artery Anomalies
Aberrant Origin of Coronary Artery (Right or Left) from the Aortic Root
Anatomy
Anomalous aortic origin of a coronary artery (AAOCA) from the opposite sinus of Valsalva has been associated with myocardial ischemia, ventricular arrhythmias, and sudden death, particularly when the vessel courses between the great arteries (Fig. 14.1) [28, 31–49]. Although AAOCA from the non-coronary, or posterior sinus of Valsalva has been described, it is quite rare and usually not associated with myocardial ischemia or sudden death [32]. Similarly, AAOCA can occur from the opposite sinus of Valsalva (either the right coronary arising from the left sinus or the left coronary arising the right sinus of Valsalva) but is usually not associated with myocardial ischemia unless the anomalous coronary courses in between the great arteries [36, 39]. When the anomalous coronary is interarterial, it can course within the myocardial sulcus between the great arteries (intramyocardial) or within the anterior wall of the aorta between the great arteries (intramural) [28, 50]. AAOCA of the left coronary artery from the right sinus of Valsalva with the anomalous vessel coursing between the great arteries is quite rare (with an estimated incidence of 0.03–0.05 %), however, this is frequently associated with sudden cardiac death [31–39]. AAOCA of the right coronary artery from the left sinus of Valsalva is more common (incidence estimated 0.1 %) and is also associated with sudden cardiac death [41–49].
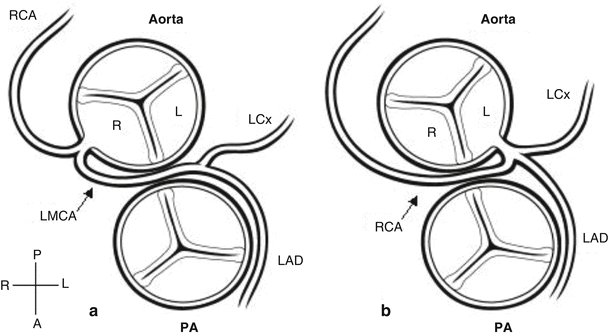

Fig. 14.1
Schematic diagram of the two forms of anomalous origin of a coronary from the opposite sinus potentially associated with myocardial ischemia. Panel a anomalous origin of the left coronary artery from the right sinus of Valsalva; Panel b anomalous origin of the right coronary artery from the left sinus of Valsalva. In each case, the anomalous coronary artery can be seen coursing between the aorta and pulmonary artery (PA). With anomalous origin of the left coronary, the left main coronary artery (LMCA) arises from the right aortic sinus (R) and courses between the great arteries before dividing into its two usual branches, the left anterior descending (LAD) and left circumflex (LCx) coronary arteries. With anomalous origin of the right coronary, the right coronary artery (RCA) arises from the left aortic sinus (L) and passes between the great arteries before coursing in its usual distribution. (Orientation marker: A anterior, L left, P posterior, R right) (Reprinted with permission from Frommelt and Frommelt [86])
The mechanisms that lead to myocardial ischemia in the patient with AAOCA from the opposite sinus that courses between the pulmonary and aortic roots are unclear, but several theories have been proposed. The ostium of the anomalously arising coronary artery is frequently slit-like, possibly compromising flow reserve [32]. In addition, the anomalous coronary artery usually arises at an acute angle from the aorta, rather than perpendicularly, and this may alter flow patterns into that coronary artery bed [39]. Finally, it has been hypothesized that the interarterial course places the anomalous coronary at risk of compression between the great arteries. This seems unlikely given the low pressure in the pulmonary artery in normal individuals, even during exercise. The ischemia is more likely due to deformation of the anomalous coronary artery within the aortic wall during periods of arterial hypertension with exercise, particularly in patients with an intramural course [51].
Transesophageal Echocardiographic Evaluation
Transesophageal echocardiographic imaging of coronary artery origins is critical and should be a part of the routine complete examination in every patient. This is usually best accomplished from the mid esophageal window, using the mid esophageal aortic valve short axis view (ME AV SAX) as a starting point where both origins can be visualized by scanning superior to the aortic valve with careful interrogation of the aortic sinuses. The left main coronary artery usually arises at approximately 2 o’clock if one considers the aortic root as a clock face (using the image display noted in the American Society of Echocardiography/Society of Cardiovascular Anesthesiologists guidelines), and the right coronary artery arises at approximately 7 o’clock. Rotation of the multiplane element from the standard short axis position (0°) to 25–70°, along with slight leftward rotation of the TEE transducer, frequently improves imaging of the length of the left main coronary artery and its early branches, the left anterior descending and circumflex coronary arteries. Similarly, slight rightward rotation of the transducer, using the standard short axis view (0°), can facilitate imaging of the origin of the right coronary artery. Color Doppler flow interrogation of the left and right main coronaries is also important, as documentation of both direction and timing of flow is also helpful in diagnosing anomalies (as described below). Spectral Doppler may also facilitate this assessment.
Normal coronary artery flow is predominantly diastolic, since that is the time that aortic pressure (and thus coronary pressure) exceeds ventricular pressure. In order to identify these coronary flow signals, which are usually low velocity, the Nyquist limit of the color Doppler map should be decreased to 20–40 cm/s. Many echocardiographic systems allow presets for coronary imaging to facilitate altering the set-up to better identify the low velocity coronary flow signals.
TEE can be an important tool for prospectively identifying and/or confirming anomalous origin of the left coronary from the right sinus of Valsalva [9–11, 23, 24, 52] and anomalous origin of the right coronary from the left sinus of Valsalva [12–14, 26, 53]. Identification of either anomaly requires focused two-dimensional (2D) and color Doppler imaging of the coronary arteries. This is especially important when the course of the anomalous coronary is interarterial and intramural, because the anomalous vessel can appear to arise normally from the appropriate sinus by 2D imaging as it exits the aortic wall [22]. The anomalous intramural coronary segment can be visualized as an echo-lucent linear space in the anterior aortic wall (Figs. 14.2 and 14.3; Videos 14.1a, 14.1b, 14.2, 14.3, 14.4a, and 14.4b), but a high index of suspicion is usually necessary to recognize this during routine screening of coronary origins. The origin of the anomalous coronary artery must be carefully delineated, particularly in relationship to the aortic sinuses, since anomalous coronary artery origins with an interarterial and intramural course can arise near the commissure between the appropriate sinus and the opposite adjacent sinus. This is especially true for anomalous origin of the right coronary from the left sinus of Valsalva (Fig. 14.3; Video 14.2).
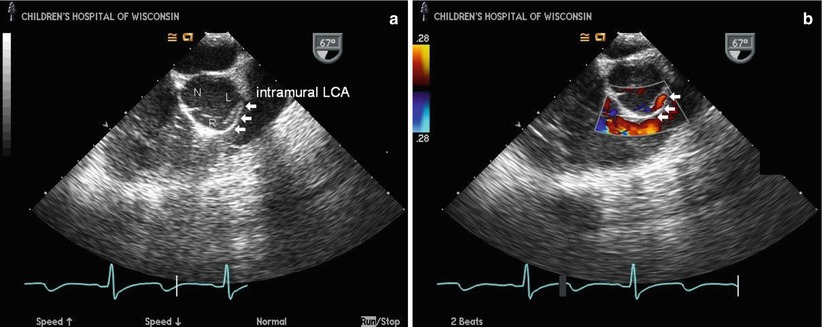
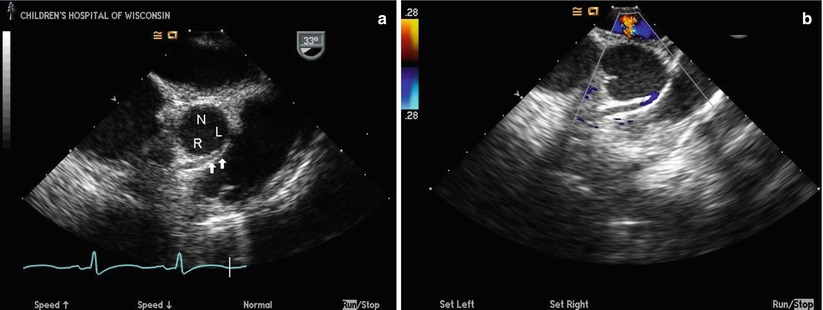

Fig. 14.2
Transesophageal echocardiogram from a mid esophageal right ventricular inflow-outflow view through the aortic root in a patient with anomalous origin of the left coronary artery from the right sinus of Valsalva with an intramural course. The two-dimensional image (a) shows the anomalous left main coronary artery (intramural LCA) arising from the right sinus of Valsalva (R) and running intramurally within the anterior aortic wall (arrows) between the aorta and right ventricular outflow tract before exiting the wall in the left sinus of Valsalva (L). The non-coronary cusp (N) is seen posteriorly. The right coronary artery origin is not visualized in this image. Color Doppler imaging (b) shows the linear diastolic flow of the anomalous coronary within the anterior aortic wall (arrows); the red color signal confirms anomalous coronary flow towards the transducer, consistent with the coronary originating from the right sinus and coursing towards the more posteriorly positioned left sinus

Fig. 14.3
Transesophageal echocardiogram from a mid esophageal aortic valve short axis view in a patient with anomalous origin of the right coronary artery (RCA) from the left sinus of Valsalva and an intramural course of the anomalous vessel. The anomalous RCA can be seen arising from the left sinus of Valsalva (L) and coursing intramurally within the anterior aortic wall (arrows) between the aorta and the right ventricular outflow tract (a) towards the right sinus of Valsalva (R). The non-coronary cusp (N) is seen posteriorly. Color Doppler imaging (b) shows the linear diastolic flow of the anomalous RCA within the anterior aortic wall; the blue color signal confirms anomalous coronary flow away from the transducer anteriorly, consistent with the coronary originating from the left sinus and coursing towards the more anteriorly positioned right sinus. The left coronary origin is not visualized
Color Doppler imaging is frequently helpful in making this diagnosis, as it can identify flow in the intramural segment. Color Doppler interrogation of the aortic root will demonstrate an abnormal linear diastolic color Doppler signal in the anterior aortic wall, representing the diastolic flow in the intramural segment. Color Doppler can also be helpful in identifying which coronary artery is anomalous. With anomalous origin of the left coronary from the right sinus of Valsalva, a red color Doppler signal will be seen in the anomalous coronary as flow moves away from the right sinus towards the more posterior positioned left sinus of Valsalva (Fig. 14.2; Video 14.4a and 14.4b). This is in contrast to anomalous origin of the right coronary from the left sinus, where a blue color Doppler signal will be seen in the anomalous coronary as flow moves away from the TEE transducer towards the right sinus from its origin in the left sinus (Fig. 14.3; Video 14.1a and 14.1b).
Other TEE views can also provide imaging evidence that a coronary artery has an anomalous origin from the aortic root. From the mid esophageal long axis view (ME LAX) through the left ventricle and aortic outflow, the anomalous coronary artery can be seen running between the great arteries as a discrete circle anterior to the aortic root as an initial clue (Fig. 14.4). Turning the probe counterclockwise from this position towards the patient’s left can allow visualization of the proximal left coronary artery branches as they leave the anterior aortic wall (Video 14.5). In addition, in patients who allow for high quality deep transgastric imaging, scanning from the aortic root more anteriorly to the pulmonary root in the deep transgastric long axis view (DTG LAX) can identify a length of the anomalous coronary artery coursing between the great arteries (Video 14.6).
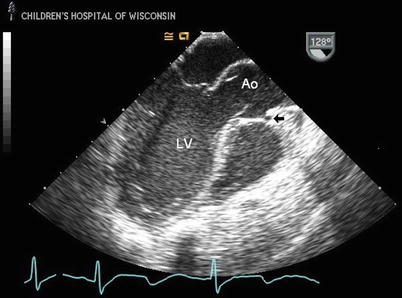

Fig. 14.4
Mid esophageal long axis image through the left ventricle (LV) and aortic root in a patient with anomalous origin of left coronary artery from the opposite sinus of Valsalva with an interarterial course. The anomalous coronary is seen coursing anterior to the aorta (Ao) between the great arteries as a discrete circle (arrow); this may be the first clue that there is a coronary anomaly, as visualization of a coronary anterior to the Ao from this view is never seen with normal coronary anatomy
Surgical repair of AAOCA has generally been reserved for patients with known symptoms of myocardial ischemia, although in some cases consideration may be given for surgical intervention in the absence of symptoms or coronary insufficiency. Multiple surgical techniques have been utilized, including coronary artery bypass grafting [54–58], patch enlargement of the anomalous coronary origin [28], reimplantation of the anomalous coronary to the appropriate sinus [59, 60], and unroofing of the intramural segment of the anomalous vessel [50, 61–64]. The unroofing procedure is performed by removing the common wall between the aorta and anomalous coronary artery to create a neo-orifice of the anomalous vessel in the appropriate sinus that arises perpendicularly, rather than obliquely, from the aortic root. The results of this technique can be easily visualized by TEE after surgery, with both imaging and color Doppler assessment of the neo-orifice (Fig. 14.5; Video 14.7).
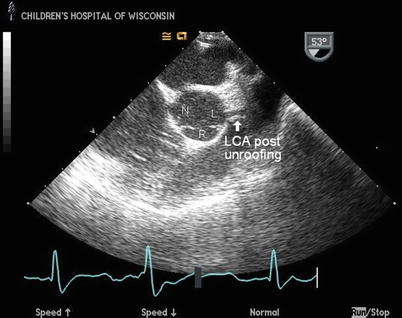

Fig. 14.5
Mid esophageal right ventricular inflow-outflow view displaying the aortic root in a patient with anomalous origin of the left coronary artery (LCA) from the right sinus of Valsalva and an intramural course of the anomalous vessel after surgical unroofing of the intramural segment. The anomalous LCA can be seen arising appropriately from the left sinus of Valsalva (L) after removal of the intramural segment with creation of large neo-orifice that is oriented perpendicular to the aortic root, similar to the appearance of a normal LCA origin. The right (R) and non-coronary (N) sinuses are indicated
Anomalous Origin of Left Coronary Artery from Main Pulmonary Artery
Anatomy
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital abnormality, occurring in approximately 1 in 300,000 children [65]. An anomalous coronary artery that arises from the pulmonary root is most commonly the left main, although anomalous origin from the right coronary artery has also been described [20, 66]. The anomalous left coronary artery typically courses adjacent to its normal aortic origin near the left aortic sinus before branching in the usual left coronary distribution. Since coronary artery flow is dependent on the diastolic pressure gradient between the vessel supplying the coronary artery and the myocardial bed it perfuses, patients with this anomaly are at risk for left ventricular ischemia. Diastolic pulmonary artery pressure is usually lower than left ventricular diastolic pressure after birth, and so patients develop a myocardial “steal” phenomenon because right coronary blood preferentially flows to the pulmonary artery instead of perfusing the left ventricular myocardium. Perfusion in the left coronary bed in all patients with ALCAPA is retrograde, supplied via collateral circulation from the normally arising right coronary artery off the higher-pressure aorta. Thus, patients with ALCAPA can develop progressive left ventricular dysfunction with signs and symptoms of myocardial ischemia/infarction. The timing of presentation during childhood is quite variable and is related to adequacy of collateralization from the right coronary artery [20]. Surgery to establish a connection of the left coronary artery with the aortic root is indicated in all patients when this lesion is identified, even in the patient with good left ventricular function, in order to normalize perfusion to the left coronary bed. Historically, the favored surgical management of ALCAPA was ligation of the left coronary artery to prevent myocardial steal. Subsequently techniques creating an aortopulmonary window and a tunnel (Takeuchi operation) that directed flow from the aortic root through the pulmonary artery to the anomalous coronary were used. Today, however, almost all patients can have direct reimplantation of the anomalous coronary artery into the aortic root, using techniques that have been refined for the arterial switch procedure for transposition of the great arteries and other coronary translocation procedures.
It should be remembered that, while most cases of anomalous origin of a coronary artery from the pulmonary artery occur with a structurally normal heart, in rare instances this entity can be seen in association with other forms of CHD such as tetralogy of Fallot.
Transesophageal Echocardiographic Evaluation
Prospective identification of ALCAPA using echocardiography has been well described, and this technique should be diagnostic in most patients [20]. Although TTE is usually adequate without the need for additional imaging, TEE can be a useful adjunct modality [67, 68]. Again, careful interrogation of the coronary artery origins and flow patterns are necessary to make the diagnosis. 2D assessment may provide direct visualization of the anomalous coronary artery insertion into the pulmonary artery from mid esophageal long and short axis imaging, with the coronary artery usually inserting into the posterior and leftward aspect of the main pulmonary artery (Fig. 14.6; Video 14.8a and 14.8b). The anomalous left coronary artery frequently courses close to its normal site of origin near the left sinus of Valsalva, however, and therefore recognition of associated echocardiographic findings is critical to the diagnosis in many patients. Because the anomalous coronary artery is perfused in retrograde fashion from the normally arising opposite coronary, spectral and color Doppler is essential. This allows for identification of retrograde filling of the anomalous coronary artery [12] and detection of abnormal diastolic flow signals in the pulmonary artery as the anomalous coronary empties into the pulmonary artery [10, 11] (Figs. 14.6, 14.7, and 14.8; Videos 14.8a, 14.8b, 14.9, 14.10, and 14.11). Color Doppler interrogation is useful in documenting the direction of flow in the coronary arteries, with the expectation that normal coronary flow is always away from, and not towards the aortic root. The normally arising coronary artery can have significant dilatation because of the obligate collateral circulation needed to perfuse the anomalous coronary system retrograde (Fig. 14.8; Video 14.9). The degree of dilatation can be dramatic in older patients who have survived with this anomalous coronary circulation for several years. As in all cases of suspected coronary artery anomalies, additional echocardiographic parameters to be obtained include the assessment of global and segmental ventricular systolic function. In addition, atrioventricular valve competency should be assessed as children with ALCAPA can have various degrees of mitral regurgitation resulting from papillary muscle ischemia or infarction. The analysis of regional ventricular function is facilitated by the transgastric mid short axis (TG Mid SAX) and mid esophageal four chamber (ME 4 Ch) views. These TEE windows allow for examination of myocardial segments supplied by the right, circumflex, and left anterior descending coronary arteries from cross sections at the basal, mid, and apical ventricular levels (refer to Chap. 5). This analysis is important not only in the pre-cardiopulmonary bypass setting, but also immediately following the surgical intervention.

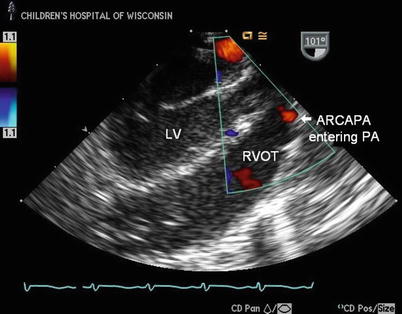
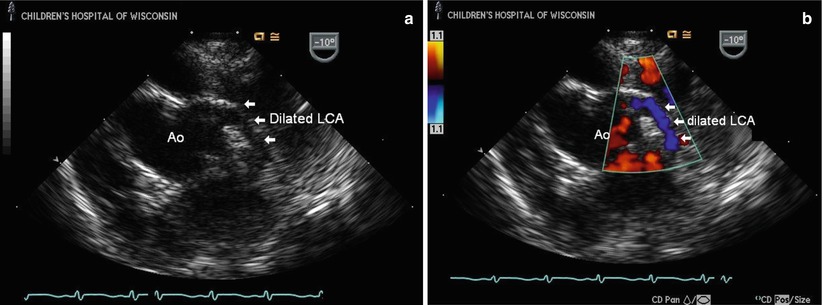

Fig. 14.6
Mid esophageal aortic valve short axis TEE images of the anomalous insertion of the left main coronary artery (LCA) into the posterior aspect of the proximal main pulmonary artery (PA) in a patient with anomalous left coronary artery from the pulmonary artery (ALCAPA). In (a) the LCA is seen entering the PA through a short coronary segment and then bifurcating into the left anterior descending and circumflex branches (arrows), clearly a long distance from the normal entrance into the aortic root (Ao). Color Doppler interrogation in (b) shows a blue color signal in the LCA (arrows), consistent with coronary flow towards the PA, indicating retrograde filling of the anomalous vessel that empties into the PA

Fig. 14.7
Mid esophageal long axis image through the left ventricle (LV) in a patient with anomalous origin of the right coronary from the pulmonary artery (ARCAPA). There is a diastolic color Doppler flow signal entering the pulmonary artery (PA) anteriorly above the right ventricular outflow tract (RVOT). This flow signal may be the first clue that there is an anomalous coronary arising from the PA, as direct visualization of a coronary entering the PA can sometimes be difficult to obtain

Fig. 14.8
Transesophageal images at the level of the mid esophagus displaying the aortic root (Ao) and a dilated left coronary artery (LCA) in a child with anomalous right coronary artery (RCA) from the pulmonary artery; the LCA measured 3.5 mm at its origin, which is significantly abnormal for age. (a) The LCA arises normally from the Ao; the anomalous RCA is not seen. Color Doppler documents flow in the dilated LCA, even without lowering the Nyquist limit (b), due to the increased flow into the left coronary bed as it functions as the single vessel supplying the entire myocardium with steal of coronary flow from the anomalous RCA emptying into the low pressure pulmonary bed
Coronary Artery Fistula
Anatomy
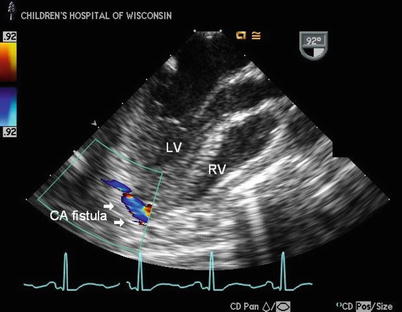
Congenital coronary artery fistula (CAF) is a rare isolated anomaly of the coronary artery system defined as a direct communication between a coronary artery and another vascular structure or cardiac cavity. The involved coronary artery usually arises normally from the aorta and connects to one of the intracardiac chambers, systemic veins, or pulmonary artery via a tortuous anomalous branch. It more frequently involves the right coronary artery and the fistula usually drains into one of the right heart chambers [69]. Symptoms and signs are dependent on the size of the fistulous connection; rarely, large fistulas can have a significant left-to-right shunt with resultant congestive heart failure and cardiomegaly in infancy [70]. Surgical closure is safe and effective [71, 72]. Recent advances in interventional techniques now allow closure of CAF in the cardiac catheterization laboratory using devices such as detachable coils and balloons, and this has become the initial treatment of choice in children and adults [73–75].
Transesophageal Echocardiographic Evaluation
TTE is usually diagnostic and can identify the involved coronary artery and its site of drainage, but many reports exist detailing the value of TEE in diagnosing coronary fistulae [76–82]. The hallmark is identifying the site of drainage of the fistula into the heart or vascular bed (Fig. 14.9; Video 14.12); a continuous turbulent jet of flow is usually present where the fistula empties (since all chambers except the aorta and left ventricle are at lower pressures than the coronary bed). Color Doppler is critical in identifying the site of drainage of the fistula. The entire course of the coronary fistula can sometimes be tracked using imaging and color Doppler interrogation and the site of drainage is best identified by color Doppler because a high velocity continuous color signal is evident where the coronary artery empties into the usually lower pressure chamber. Because of low resistance into the affected coronary artery secondary to steal of flow through the fistula as it empties into the lower pressure chamber where it terminates, the coronary artery supplying the fistula will be dilated (Fig. 14.10). The coronary artery can be quite dilated, ectatic, and tortuous, depending upon the amount of fistula flow (Figs. 14.11 and 14.12; Videos 14.13a and 14.14a), and results in easily identifiable flow in the vessel by color Doppler (Videos 14.13b and 14.14b).