, Domenico Corrado2 and Cristina Basso1
(1)
Cardiovascular Pathology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
(2)
Cardiology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
7.1 Preexcitation Syndromes
In the normal heart, the electrical impulse arises from the sinus node, and it is transmitted from the atria to the ventricles only through the Tawarian AV conduction axis (i.e., AV node, His bundle, and bundle branches) and Purkinje fibers, after a slowdown at the AV node, which allows the atria to empty during ventricular diastole. The AV node is located at the AV septal junction, in front of the coronary sinus and underneath the membranous septum. It consists of specialized myocardium with low speed of electrical transmission and long refractory period [1–4].
There are diseases in which the AV conduction may occur also outside the specialized axis, thus bypassing the AV node.
In Wolff–Parkinson–White syndrome, characterized by short PR interval with delta wave at the basal ECG and episodes of supraventricular tachycardia [5], there is one or more accessory pathways (also known as “Kent fascicle”), which connect directly the atrial to the ventricular myocardium, outside the specialized AV axis (Figs. 7.1 and 7.2) [6–8]. The accessory pathways consist of working myocardium, which does not delay the impulse transmission from the atria to the ventricles, because it does not possess the decremental properties typical of the specialized myocardium. Thus, the electrical impulse transmission, through the accessory pathway, may anticipate the depolarization of part of the ventricular myocardium (delta wave). It also may allow the reentry of the electrical ventricular depolarization wave to the atria, triggering a circuit of supraventricular tachycardia. If the period of refractoriness of these accessory pathways is short, the risk is that a lone atrial fibrillation may transmit the electrical impulse very fast to the ventricles (up to 1 to 1) through the anomalous bundle, thus converting into ventricular fibrillation. This is why patients affected by Wolff–Parkinson–White syndrome are at risk of SCD [9–13].
The anomalous bundle connecting the atrial to ventricular musculature is located at both the right and left AV rings, particularly in correspondence of the posterior “mural” mitral leaflet [6–8].
The bundle is thin (100–150 μ in thickness) and located close to the fibrous ring, not so far from the ventricular cavity, as to be easily ablated from the endocardial side (Fig. 7.3). Serial histological section investigation of the lateral AV ring or AV septum is needed to detect this congenital anomaly [14], which is considered the smallest structural heart defect at risk of SCD [8].
In Lown–Ganong–Levine syndrome, ventricular preexcitation occurs along the regular AV conduction axis and is characterized by a short PR interval in the absence of delta wave [15]. The AV conduction is accelerated (“enhanced AV conduction”) because of a congenitally hypoplastic AV node [16] or of the existence of an anomalous pathway by passing the AV node (site of slow down) and connecting directly to the His bundle (Figs. 7.4 and 7.5) [2–4]. These atrio-hissian anomalous pathways (James or Brechenmacher’s fascicles) consist also of working myocardium, which has high-speed properties for impulse conduction and short refractory period, allowing both preexcitation and reentry tachycardia, as well as triggering ventricular fibrillation in the occurrence of atrial fibrillation [1–4].
Anyway, in the presence of ventricular preexcitation, the trigger of atrial fibrillation, degenerating into ventricular fibrillation, remains intriguing. Episodes of supraventricular tachycardia, which occasionally turns into ventricular fibrillation, have been hypothesized. Furthermore, atrial myocarditis, possibly triggering paroxysmal atrial fibrillation, has been demonstrated in young people dying suddenly (Fig. 7.6) [8]. In both conditions, the anomalous pathway of working myocardium should possess a very short refractory period to convert atrial fibrillation into ventricular fibrillation.
7.2 Atrioventricular Block
The AV block in the young, at risk of asystole and cardiac arrest, may be congenital or acquired. Among congenital blocks, the most frequent is the one observed in children of mother with systemic lupus erythematosus [17, 18]. Both the sinus node and AV node appear destroyed by an inflammatory necrotic process, most probably immune in origin, with lymphocytic infiltrates, fibrosis, and even calcification. The other congenital non-Lupus-related AV block is the consequence of a maldevelopment of the specialized AV axis, with a lack of connection between the AV node and the His bundle. It may be observed in otherwise normal hearts or in hearts with complex congenital heart diseases, like congenitally corrected transposition and single ventricle [19–21].
The acquired AV block, which appears early in young age, presents a quite different substrate (Lenegre’s disease) [22]. The histopathology consists of a fibrosis of the bifurcating bundle and proximal left and right bundle branches, whereas the surrounding working myocardium appears intact (Fig. 7.7). A cardiomyopathy of the specialized conducting tissues has been postulated [23].
Some of these cases with acquired AV block are heredofamilial, and mutations in the SCN5A gene encoding sodium ion channel have been discovered through molecular investigations (Fig. 7.8) [24]. By the way, Brugada syndrome, which is also explained by SCN5A mutations, is characterized by PR prolongation and right bundle branch block, which at histological examination appears to have an organic substrate, i.e., fibrosis of the bifurcating bundle and proximal right bundle branch [25].
7.3 Cardiac Tumors
Cardiac tumors are rare and might exceptionally cause SCD due to an either mechanical (for instance, endocavitary myxoma or rhabdomyoma with hemodynamic compromise – see Chap. 3) or arrhythmic pathophysiologic mechanism [26, 27].
As far as the latter group is concerned, we will take into consideration cystic tumor of the AV node, Purkinje cell tumor, and cardiac fibroma, which are reported as a cause of SCD in the young.
The cystic tumor of the AV node (also known as Tawarioma or celothelioma of the AV node) represents an embryonal inclusion in the heart’s center of mesodermal tissue and is characterized by heterotopic epithelial replacement of the AV node with multicystic appearance. It grows more or less rapidly with tubule-cystic features as to produce congenital, juvenile, or adult complete supra-hissian AV block [26, 28] (Fig. 7.9).
The Purkinje cell tumor (also known as histiocytoid cardiomyopathy, idiopathic infantile cardiomyopathy, purkinjoma) is a rare cause of severe and intractable tachyarrhythmias early in infancy [26, 29–33]. The masses grossly appear as focal yellowish nodules or areas of discoloration, ranging in size from 1 mm to 1.5 cm in diameter, most commonly located along the conduction system and within the working myocardium of the left ventricle. Microscopically, these masses contain large oval cardiac myocytes with a coarse granular pale cytoplasm (Fig. 7.10).
Cardiac fibroma is the second most frequent type of cardiac tumor in the pediatric age. Macroscopically, the fibroma usually is a single, solid, well-defined, whitish, and whorled not encapsulated mass, almost invariably intramural, and usually located in the right or left ventricular free walls, or in the interventricular septum. At histology, the fibroma consists of a homogeneous mass of fibroblasts mixed with abundant extracellular matrix, which often entraps cardiomyocytes. These tumors may reach huge dimensions, even up to 8 cm of diameter, as to obstruct the ventricular cavity and cause hemodynamic symptoms/signs. When located in the central fibrous body, the fibroma might compress the His bundle and induce AV block [34, 35]. However, fibroma often accounts for life-threatening ventricular arrhythmias due to reentry of the intraventricular electrical impulse.
7.4 Image Gallery
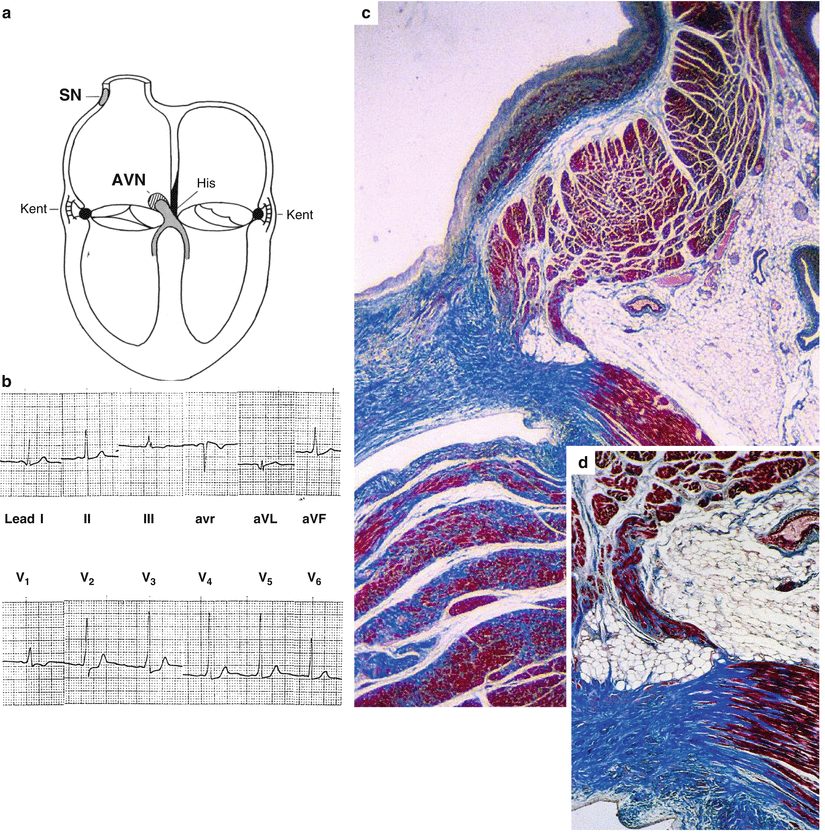
Fig. 7.1
Arrhythmic cardiac arrest in a 35-year-old man with an in vivo diagnosis of Wolff– Parkinson–White syndrome. (a) Diagrams illustrating the normal specialized sinus node (SN) and atrioventricular node (AVN) and junction as well as the accessory AV connections (Kent fascicles) which may be situated at the septal and lateral levels; (b) 12-lead ECG tracing with short PR interval and delta wave; (c) the Kent fascicle, located close to the endocardium, joins the working atrial and ventricular myocardium (Heidenhain trichrome); (d) close-up of (c)
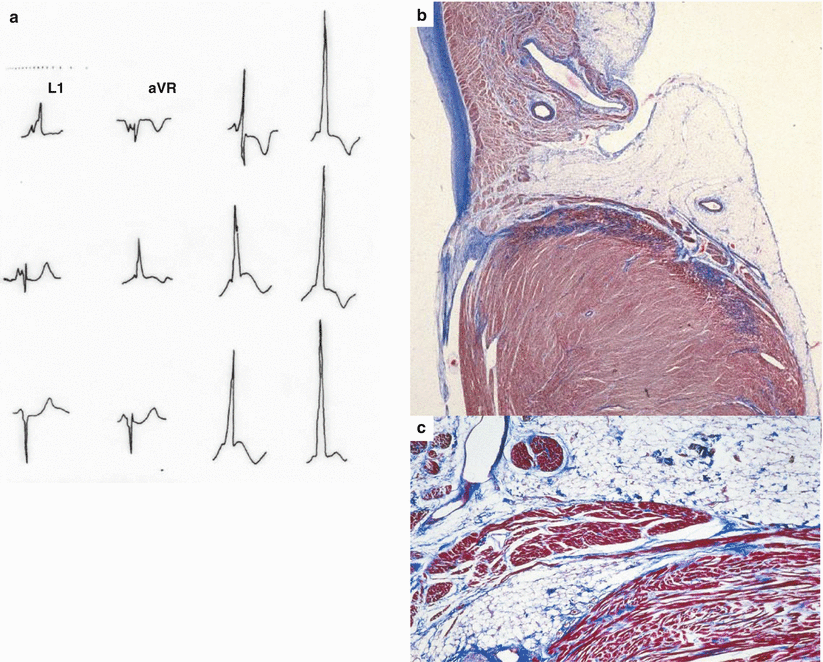
Fig. 7.2
Arrhythmic cardiac arrest in a 9-year-old boy with an in vivo diagnosis of Wolff– Parkinson–White syndrome. (a) 12-lead ECG tracing with short PR interval and delta wave. (b) A section of the left lateral AV ring shows the Kent fascicle, joining the working atrial and ventricular myocardium (Heidenhain trichrome); (c) close-up of (b)
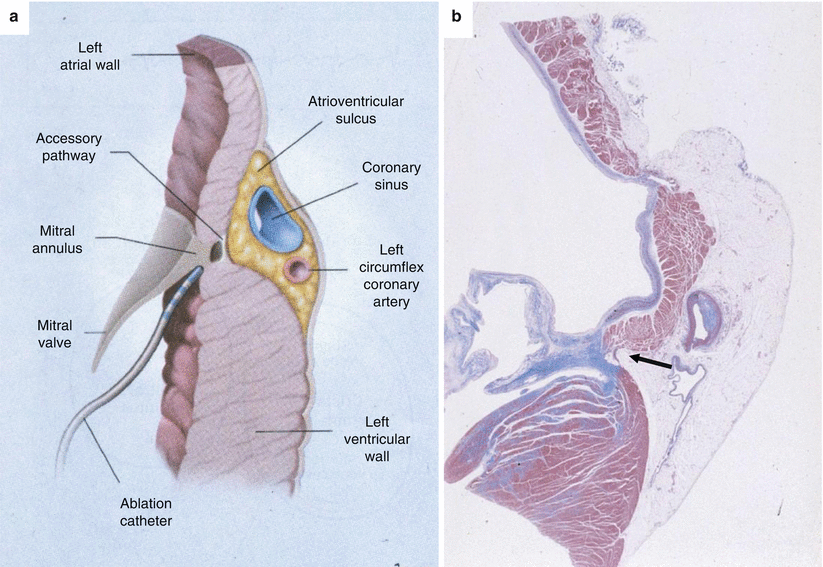
Fig. 7.3
(a) Diagram illustrating the left AV junction. The location of the accessory AV connection (Kent fascicle) close to the endocardium is easily reachable by the ablation catheter. (b) Panoramic histology note the Kent fascicle located close to the endocardium (arrow) (same case as Fig. 7.1) (Heidenhain trichrome)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


