4 Complications of Percutaneous Coronary Interventions
Percutaneous coronary intervention (PCI) is associated with rare but serious complications. Most of the complications are generic to all diagnostic coronary angiography procedures and some are specific to coronary intervention. Events like death, myocardial infarction (MI), and bleeding occur at higher rates for interventional procedures since there is prolonged procedural time, complexity, and the use of anticoagulation (see Tables 4-1 and 4-2). It is critical to understand the possible complications of PCI in order to provide proper informed consent to the patient. It is also critical to be vigilant and to recognize potential complications at an early stage to try to reverse the adverse outcome, as the most common cause of all post-PCI deaths is from a procedural complication rather than from a preexisting cardiac condition. Fortunately, death is very rare with diagnostic angiography (<0.1%). The mortality rate increases 13 times with the addition of the complexity of coronary intervention to 1.3% (see Table 4-3).
Table 4-1 Event Rates of Diagnostic Versus PCI Complications
| Complication | Event Rate Diagnostic Procedure (%) | Event Rate Interventional Procedure (%) |
|---|---|---|
| Death | 0.1 | 1.3 |
| Significant bleed | 0.5 | 5–12 |
| AV fistula | 0.75 | 1.1 |
| Pseudoaneurysm | 0.2 | 1–2 |
| Contrast-induced nephropathy | 5 | 8–57 |
| Periprocedural MI (>3xULN cardiac enzyme) | 0.1 | 8 |
| Air embolism | 0.1–0.3 | 0.1–0.3 |
| Cerebrovascular accident | 0.3 | 0.3 |
| Ventricular arrhythmia | 0.4 | 0.84 |
| Coronary dissection | .03–0.46 | 29–50 |
| Aortic dissection | <.01 | .03 |
| Infection/bacteremia | .11 | 0.64 |
| Anaphylactoid reaction to contrast | 0.23 | 0.23 |
| Cholesterol embolization | 0.8–1.4 | 0.8–1.4 |
AV, arteriovenous; MI, myocardial infarction; ULN, upper limits of normal.
Table 4-2 Complications Specific to PCI
| Complication | Event Rate (%) |
|---|---|
| No-reflow phenomenon | 2 |
| Stent thrombosis | 2 |
| Vessel perforation | 0.84 |
| Stent embolization | 0.4–2 |
| Need for emergent bypass surgery | 0.15–0.3 |
| Wire fracture | 0.1 |
| Stent infection | <.1 (case reports only) |
Table 4-3 Modes of Death During PCI
| Mode of Death | Event Rate (%) |
|---|---|
| Low output failure | 66.1 |
| Ventricular arrhythmias | 10.7 |
| Stroke | 4.1 |
| Preexisting renal failure | 4.1 |
| Bleeding | 2.5 |
| Ventricular rupture | 2.5 |
| Respiratory failure | 2.5 |
| Pulmonary embolism | 1.7 |
| Infection | 1.7 |
Adapted from Table 1, page 633 of Malenka DJ, O’Rourke D, Miller MA et al. Cause of in-hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. Am Heart J 1999; 137(4):632-638.
Vascular Access
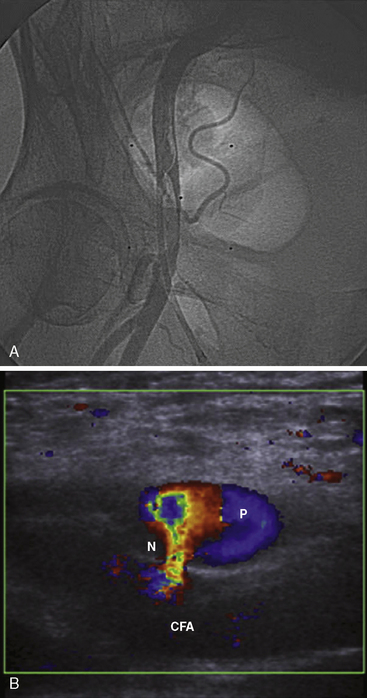
The first part of any PCI begins with vascular access. Using the femoral access, the major complications are femoral artery dissections (Fig. 4-1A, B), pseudoaneurysm, arteriovenous (AV) fistula, and retroperitoneal bleeding. As seen in Table 4-1, the incidence of these complications is increased compared to a strictly diagnostic procedure. All arterial complications are markedly reduced using the radial artery access.
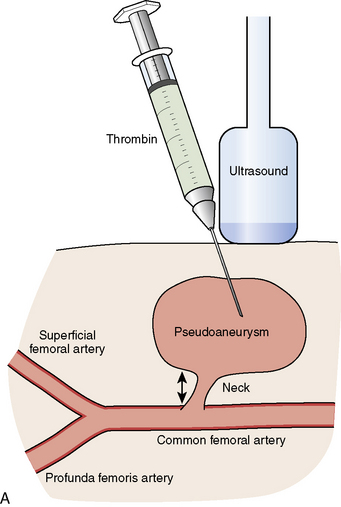
A femoral artery pseudoaneurysm represents failure of sealing of the initial arterial puncture site, allowing arterial blood to flow into the surrounding tissue. This forms a pulsatile hematoma that acts as the covering roof of the aneurysm (see Fig. 4-1A, B). Pseudoaneurysms are late appearing, associated with local pain and swelling and diagnosed with femoral ultrasound with an excellent sensitivity of 94% to 97%. There are multiple risk factors for pseudoaneurysm (see Table 4-4).
Table 4-4 Risk Factors for Pseudoaneurysm Formation
| Procedural Factors |
| Catheterization of both artery and vein |
| Cannulation of the superficial femoral or profunda femoris rather than common femoral |
| Inadequate compression post procedure |
| More anticoagulation used |
| Patient Factors |
| Obesity |
| Hemodialysis |
| Calcified arteries |
Small pseudoaneurysms (<2 cm) often close spontaneously within 1 month. In larger pseudoaneurysms, or in small ones that fail to close, active treatment is necessary. The two most common treatment methods are ultrasound guided compression or thrombin injection. In some hospitals, ultrasound guided compression is not offered because of increased stress-related wrist injury to the ultrasound technician. Other advantages of thrombin injection over ultrasound compression are seen in Table 4-5.
Table 4-5 Advantages of Thrombin Injection Compared With Ultrasound Compression
| Greater technical success (96% vs. 74%) |
| Less painful to the patient and technician |
| No conscious sedation required |
| Effective in patients on anticoagulation |
| Can be used in pseudoaneurysms above the inguinal ligament |
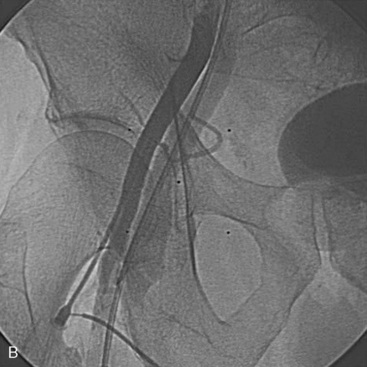
Thrombin injection can be performed by diluting 1000 U into a 1 mL syringe with normal saline (final concentration of 100 U per 0.1 mL) and injected with direct ultrasound visualization through a long 22-gauge needle until thrombus is formed in the pseudoaneurysm cavity and Doppler-detected flow is abolished (see Fig. 4-2A, B). Rarely, in very large pseudoaneurysms and those resistant to thrombin injection, vascular surgery is required.
Another complication of vascular access is AV fistula formation (Fig. 4-2A, B). This is recognized on physical exam by a palpable thrill or an audible continuous bruit. Unlike pseudoaneurysms, conservative treatment with watchful waiting is the most common treatment modality (90%). One third of persistent AV fistulae will close during the first 12 months. Most persistent AV fistulae are asymptomatic and do not require repair. Rarely, they can be symptomatic (moderate pain) and, in large patient series, about 10% of AV fistulae will ultimately require surgical repair.
Infection
An equally concerning infectious complication is the exposure of the physicians or staff to the patient’s potential pathogens. Universal precautions are to be followed by everyone in the catheterization laboratory. If there is an occupational exposure, proper management per your hospital guidelines should be followed. See Table 4-6 for U.S. Public Health Service guidelines.
Table 4-6 Management of Occupational Exposure to Hepatitis B Virus, Hepatitis C Virus, and HIV
ALT, alanine aminotransferase; HIV, Human Immunodeficiency Virus; RPR, rapid plasma reagin.
Adapted from Table 2, page 85 of Chambers C, Eisenhauer M, McNicol L, et al. Infection control guidelines for the cardiac catheterization laboratory: society guidelines revisited. Catheter Cardiovasc Interv 2006;67:78–86.
Bleeding
The last and most dangerous complication of groin access is major femoral bleeding. Large femoral hematomas have an incidence of 2.8% compared to a 0.3% incidence of retroperitoneal bleeds. A retroperitoneal hematoma or a significant femoral hematoma (>5 cm) often requires blood transfusions and prolonged hospitalization. More significant bleeds can require surgery, and significant bleeding in relation to PCI has been shown to correlate with mortality. Significant risk factors for major femoral bleeding are listed in Table 4-7.
Table 4-7 Significant Risk Factors for Major Femoral Bleeding
| Risk Factor | Odds Ratio |
|---|---|
| Age >75 vs. <55 | 2.59 |
| Heparin use postprocedure | 2.46 |
| Severe renal impairment | 2.25 |
| Age 65–74 vs. <55 | 2.18 |
| Female patient | 1.64 |
| Closure device use | 1.58 |
| Sheath size 7F–8 F vs. <6 | 1.53 |
| GP IIb/IIIa use | 1.39 |
| Longer procedure duration | 1.2 |
Adapted from Doyle B, Ting HH, Bell MR, et al. Major femoral bleeding complications after PCI. JACC Cardiovasc Interv 2008;1(2):202–209.
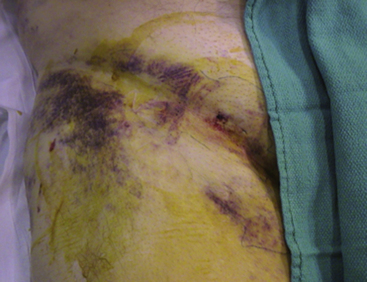
The bleeding complication of most concern is a retroperitoneal hematoma, as large amounts of blood can fill the pelvic cavity and shock can develop rapidly. If a retroperitoneal bleed is suspected (see Table 4-8), volume (crystalloid solutions) should be given and blood should be ordered immediately for transfusion as soon as available. A vascular surgeon should also be consulted immediately. If the patient remains hemodynamically unstable despite volume resuscitation, surgery or endovascular repair (covered stent placement) may be needed; this occurs in approximately 16% of patients. However, the majority (84%) of cases can undergo a conservative “watchful waiting” strategy, as the hematoma usually stabilizes from tamponade of the initial site of extravasation. A computed tomography (CT) scan will be confirmative of the clinical diagnosis and should only be ordered once the patient is stable. Vascular surgeons use the CT scan as a baseline study and as a method to localize the origin of the bleed (if radiographic contrast media is used). Most retroperitoneal hematomas are caused by bleeding from the external iliac artery above the inguinal ligament or inferior epigastric artery. Rarely, bleeding below the inguinal ligament can track between tissue planes and extend into a retroperitoneal accumulation. Bleeding might also rarely extend to the scrotum through extension along the spermatic cord. Most cases of scrotal hematoma can also be managed conservatively with elevation and ice. However, rarely, large tense scrotal hematomas can cause significant pain and may compromise the viability of the scrotal skin and/or testicle which would require urgent surgical exploration (Fig. 4-3).
Table 4-8 Classical Signs and Symptoms of Retroperitoneal Bleed
| Hypotension |
| Bradycardia |
| Back/flank pain |
| Groin pain |
| Abdominal pain |
| Transient response to fluid loading |
| Grey Turner sign (bruising along flank) [late appearing] |
| Cullen sign (bruising around umbilicus) [late appearing] |
Complications of Vascular Access Closure Devices
Atheroembolism
Peripheral atheroembolism with obstruction of small arteries and arterioles by cholesterol crystals is known to produce the cholesterol embolization syndrome (CES), a rare occurrence (incidence of 0.75%–1.4%) using the above precautions. Cholesterol emboli are diagnosed by one of three cutaneous signs (see Table 4-9 and Fig. 4-4) and an elevated eosinophil count. In-hospital mortality is as high as 16% in those patients with definite CES, as multiorgan embolization often can lead to multiorgan failure.
Table 4-9 Cutaneous Signs of Cholesterol Embolization Syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree