Complications of Myocardial Infarction
Despite advances in technology and therapeutics, in-hospital mortality following acute myocardial infarction (AMI) remains high. The leading cause of death in these patients is cardiogenic shock (CGS), with an incidence of approximately 7%.1 CGS in the AMI setting can result from severe left ventricular (LV) or right ventricular (RV) systolic dysfunction, dynamic left ventricular outflow tract (LVOT) obstruction, or mechanical complications. These mechanical complications include acute mitral regurgitation (MR) from papillary muscle rupture, tamponade from cardiac free wall rupture, and left-to-right shunting from a ventricular septal defect (VSD). Arrhythmias and inflammatory sequelae are most often less deleterious unless they occur in an unmonitored setting or in a patient who is already in shock or hemodynamically tenuous.
LEFT VENTRICULAR DYSFUNCTION COMPLICATED BY CARDIOGENIC SHOCK
Isolated LV failure accounts for the majority of shock cases following AMI. In the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) Trial and concurrent SHOCK Trial registry, predominant LV failure was the cause of CGS in 78.5% of the patients, and in-hospital mortality was 59.2%.2 Autopsy studies have shown that those patients who develop LV failure have lost approximately 40% of their myocardial mass to necrosis.3 This generally occurs after a large transmural myocardial infarction (MI) or following a relatively small, nontransmural event in a patient with prior ischemic damage. In the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) Trial that examined outcomes associated with thrombolytic therapy in 41,021 patients with acute ST-elevation myocardial infarction (STEMI), risk factors for the development of shock were advanced age, female gender, prior infarction, anterior infarction location, and diabetes mellitus.4
Symptoms and Signs
Upon presentation, subjective symptoms usually do not clarify the diagnosis; however, early recognition of symptoms of malperfusion can be critical. The physical examination can be helpful. One of the most popular systems to categorize these patients is the Killip classification (Table 42.1). Shock is present if there are signs of inadequate tissue perfusion with or without hypotension, which is defined as a systolic blood pressure <90 mm Hg. Signs of inadequate tissue perfusion include altered mental status, oliguria, and chemical evidence of end-organ damage such as a rise in serum creatinine, lactate, and/or liver transaminases. Patients are often tachycardic, hypothermic, and have cool extremities. Neck vein distention can be seen, and an S3 gallop is sometimes heard on physical examination. Peripheral edema and displacement of the point of maximal impulse is less likely unless it existed prior to the AMI.
TABLE
42.1 Killip Classification
Diagnosis
In addition to the symptoms and physical findings mentioned above, the electrocardiogram (ECG) is critical in diagnosis. Anterior STEMI is the most common cause of shock in the AMI setting.2,4 Chest x-ray (CXR) can demonstrate an enlarged cardiac silhouette, although this is rare in the acute phase if prior myocardial damage has not occurred. Varying degrees of pulmonary venous congestion and edema can be seen. Transthoracic echocardiography (TTE), though not necessary for diagnosis, should be performed urgently in patients with shock or signs of congestive heart failure (CHF) to assess the extent of LV and/or RV dysfunction and exclude mechanical complications. Right heart catheterization can also be used to confirm the diagnosis and monitor therapy. In CGS, the cardiac index is typically <2.2 L/min/m2; the mixed venous oxygen saturation is low, typically <65%; and the pulmonary capillary wedge pressure (PCWP) is elevated, typically >18 mm Hg.
Treatment
Treatment involves a combination of revascularization, medication, and mechanical therapy. It is important to remember that mechanical complications other than isolated LV dysfunction must be ruled out in the initial phases of management. Although this can be done with TTE, transport to the catheterization laboratory and implementation of aggressive supportive measures such as placement of an intra-aortic balloon pump (IABP) should not be delayed for this purpose. It is an American College of Cardiology/American Heart Association (ACC/AHA) Class I recommendation that intra-arterial blood pressure monitoring and a Class IIa recommendation that right heart catheterization should be used to monitor patients in CGS.5
REVASCULARIZATION
Thrombolytic therapy has a limited effect on the high mortality rate associated with AMI complicated by CGS.2,4 Data to support this come from the Italian Group for the Study of Streptokinase in Myocardial Infarction (GISSI), which randomized >11,000 patients with AMI to thrombolytic therapy versus no thrombolytic therapy. In both groups, the subset of patients with CGS had a 70% mortality rate.6 Fortunately, with the advent of more aggressive and invasive strategies for the management of AMI, survival of patients with AMI complicated by CGS has improved. Although the SHOCK Trial did not show a significant reduction in 30-day mortality between patients undergoing early revascularization and those receiving initial medical stabilization, 6-month and 1-year survival were both significantly improved in those who underwent early revascularization, defined as within 18 hours of the diagnosis of shock.2,7 Only one subgroup in this study, those 75 years of age or older, did not benefit from early revascularization at 6 months and 1 year. It is therefore an ACC/AHA Class I recommendation that patients younger than 75 years of age with an acute STEMI (including new-onset left bundle branch block) complicated by CGS undergo early revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).5 This recommendation applies only to patients who present within 36 hours of MI onset and who can be revascularized within 18 hours of shock development. This recommendation is Class IIa for selected patients who are 75 years of age or older if they have good functional status. Therefore, treatment must be individualized in this group of patients. Whether shock is present upon arrival or manifests during the course of hospitalization, clinicians are charged with developing protocols to transfer patients to STEMI-referral centers in the event of CGS (Class I recommendation per ACC/AHA).8
Although the use of thrombolytic therapy has limited efficacy on the high mortality rate associated with this condition, it is acceptable in two circumstances. The first is when the patient is not a candidate for either surgical intervention or PCI. The second is when PCI or CABG is expected to be delayed. For specific recommendations regarding thrombolytic, interventional, and surgical strategies following AMI.
MEDICAL AND MECHANICAL THERAPIES
Although prompt cardiac catheterization and percutaneous or surgical revascularization are the primary goals in patients with AMI complicated by CGS, measures to stabilize the blood pressure and achieve adequate tissue perfusion must begin immediately. If the patient does not possess signs of volume overload, therapy can begin with rapid volume expansion. If this is not corrective, or the patient has volume overload, vasopressor therapy becomes necessary. Dopamine is the drug of first choice if systolic blood pressure is more than 70 mm Hg and <100 mm Hg, and norepinephrine should be added if the patient remains hypotensive or has signs of inadequate tissue perfusion on maximum doses. It is acceptable to start with norepinephrine if the patient’s blood pressure is <70 mm Hg, with an attempt to transition to dopamine once the patient’s systolic blood pressure improves.5 Epinephrine and phenylephrine are not first choices in CGS because of their significant α-agonist activity, which can lead to an increase in afterload and subsequent decrease in cardiac output. They may become necessary, however, if dopamine and norepinephrine fail to stabilize the patient. Dobutamine can also be used in patients with acute pulmonary edema with systolic blood pressure >70 mm Hg and <100 mm Hg.
Short-Term Circulatory-Assist Devices
In the reperfusion era, a large randomized trial and two large registries have shown benefit of IABP use in patients with AMI complicated by CGS. In GUSTO-I, early insertion of an IABP in conjunction with thrombolytic therapy demonstrated a trend toward lower 30-day and 1-year mortality, though the bleeding risk was higher.9 This trial also demonstrated that patients who received no or late IABP therapy had a much higher mortality within the first 8 hours of hospitalization than those receiving IABP therapy early In the SHOCK Trial Registry and the second National Registry of Myocardial Infarction, patients who received both IABP therapy and thrombolytic therapy had a significant mortality benefit over patients who received thrombolytic therapy alone.10,11 In addition, those who received early IABP and thrombolytic therapy in the SHOCK Trial Registry had a higher likelihood of receiving revascularization. Early IABP therapy, therefore, appears to have a mortality benefit in patients with CGS complicating AMI. This is why insertion under these circumstances remains an ACC/AHA Class I recommendation, provided no contraindications exist. These contraindications are aortic dissection, more than moderate aortic regurgitation, sepsis, bleeding diathesis, iliac or aortic atherosclerosis that impairs lower extremity blood flow, patent ductus arteriosus, and an anatomic abnormality of the femoral artery, iliac artery, or aorta that prevents insertion.12 IABP insertion should be performed concomitant with the early stabilization efforts discussed previously. Once in place, it may allow for weaning of both vasopressor and inotropic agents, which increase myocardial workload and are therefore not ideal in the AMI setting.
If an IABP is felt to be insufficient in producing adequate cardiac support, there are several options for patients who present with CGS with LV dysfunction and severe MR (similar to indications for IABP). Impella—a device with the inlet end in the LV and output in the aorta with its body through the aortic valve—has been developed where the blood is pumped directly from the LV into the aorta with the use of a microaxial pump in the Impella catheter that’s controlled by an external console. Currently, two types of Impella are available: 2.5 and 5.0 corresponding to the maximum cardiac output produced by the device. The advantage of this device is its ability to be placed in the catheterization laboratory much like an IABP with higher cardiac output produced. A major disadvantage is the size of the sheath necessary to place the catheter, which is larger than those used for IABP placement. Preliminary studies have shown that the Impella device can be placed safely and might lead to improved aortic and coronary pressures with decreased coronary microvascular resistance.13,14 A small randomized trial comparing the 2.5 device with IABP in the setting of CGS post-MI has shown improved hemodynamics at 30 minutes; however, it has failed to show better survival compared to IABP.15 The Impella 5.0 device can provide larger cardiac outputs in patients when necessary; however, data are not yet available regarding outcomes. As such, no recommendations for the use of these devices are yet in the guidelines.
The Tandem Heart—a device where inflow cannula is placed in the left atrium via transseptal puncture using a venous access site and outflow cannula in the femoral artery—allows bypassing of the left ventricle with the use of a centrifugal pump. This device can provide up to 5.0 L/min of cardiac output in tandem with the heart when used percutaneously. While the Tandem Heart provides higher outputs produced than those by IABP and the Impella 2.5, the transseptal approach requires more advanced operator training which can be a limiting factor. In a small randomized study of patients with CGS in the setting of MI, use of the Tandem Heart was associated with improved hemodynamics compared to the IABP; however, mortality at 30 days was no different. Such improved hemodynamics come with a cost of increased bleeding and limb ischemia, however.16
There are no studies comparing the Tandem Heart with the Impella device, but the above studies have shown that when a higher level of support is necessary; both devices perform better than the IABP hemodynamically, and may be more beneficial in the critically ill when a higher level of cardiac support is necessary. Potentially higher vascular complication rates necessitate that experienced operators perform these procedures.
Longer-Term Circulatory-Assist Devices
Decisions regarding placement of circulatory-assist devices in the AMI setting, other than the short-term devices noted above, should involve collaboration with a cardiothoracic surgeon skilled in their placement and follow-up. In addition, the medical and surgical cardiac transplantation teams should be consulted. Decisions regarding their placement are complex and involve multiple considerations in addition to the hemodynamic status of the patient. Mechanical cardiac support devices in AMI complicated by isolated LV dysfunction should be considered when patients remain hemodynamically unstable and have evidence of end-organ hypoperfusion despite IABP therapy and maximal inotropic support. In addition, surgically correctable, mechanical complications of MI must be ruled out. Other issues such as transplant candidacy, potential reversibility of cardiac dysfunction, bleeding risks, expected duration of support, and the absence or presence of biventricular dysfunction are also important. In addition, the patient’s age, body habitus, and comorbid conditions must be considered.
There are a number of different circulatory-assist devices, grouped best into two categories. Extracorporeal membrane oxygenation (ECMO) is a device where the patient’s circulatory and respiratory system is fully supported by way of providing cardiac support and oxygenation of the blood externally. ECMO allows for temporizing the acute hemodynamic and respiratory decline while further decisions can be made as the patient is evaluated. If a patient is subsequently refused for transplantation, he or she can be weaned from the device and removal can occur without reinstitution of cardiopulmonary bypass. These devices are useful because they allow for observation of myocardial recovery after a period of rest and provide decision time concerning transplant candidacy. The second category of devices consists of ventricular assist devices that are suitable for long-term support. These devices are inserted via cannulation of the LV which then delivers blood to the aorta for perfusion. If a patient is deemed a transplant candidate in the acute setting, some centers will immediately place this type of device. If a short-term device was used initially, switching to a long-term device can be done as well. These devices are then explanted at the time of transplant or can be used as bridge-to-decision or destination therapies.
Vasodilators
Vasodilator therapy is ideal in patients with a low cardiac output who are not hypotensive. The two drugs most frequently used in the intensive care unit (ICU) setting are nitroprusside and nitroglycerin. Nitroprusside is a direct intravenous vasodilator that produces a balanced effect on both arteries and veins. It is initiated at low doses and titrated to a mean arterial blood pressure (MAP) of 65 to 70 mm Hg. Thiocyanate and cyanide toxicity have been reported but are uncommon unless patients receive a prolonged infusion. Patients with renal dysfunction are more prone to the former, and those with liver dysfunction, the latter. Thus, nitroprusside must be used cautiously in patients with these comorbidities. Intravenous nitroglycerin is the drug of first choice in the setting of AMI and CHF. In addition to its vasodilatory effect, it has anti-ischemic properties, plus, when compared to nitroprusside, is unlikely to cause coronary steal. If a nitroprusside infusion is initiated in a patient with low cardiac output in the setting of coronary instability, nitroglycerin should also be added to negate the potential coronary steal that can be associated with nitroprusside. Both of these drugs should be avoided in hypotensive patients.
Beta-Blockers
Intravenous beta-blockers should be avoided in patients with AMI complicated by CGS (ACC/AHA Class III recommendation).17 Once the patient is hemodynamically stable and has been weaned from inotrope and IABP support, it is safe to institute low doses of oral beta-blockers.
Diuretics
Intravenous diuretics are frequently necessary in the setting of AMI complicated by CGS for the treatment of pulmonary edema and volume overload. These can cause hypotension and therefore measures to stabilize the blood pressure should be instituted before diuretics are administered to a hypotensive patient. If the patient is in extremis from a respiratory standpoint, intubation should be considered.
Aldosterone Antagonists
Long-term administration of aldosterone antagonist— eplerenone—should be considered as its use has been associated with improved survival in the post-MI setting in patients who develop heart failure.18
Angiotensin-Converting Enzyme Inhibitors and Angiotensin-II Receptor Blockers
Multiple trials studying the administration of oral angiotensin-converting enzyme inhibitors (ACE-I) during the first few days following AMI complicated by CHF have shown a decrease in both mortality and adverse cardiovascular events.19–21 Angiotensin-II receptor blockers (ARBs) have also been shown to lower mortality and adverse cardiovascular events when instituted early in patients with AMI complicated by CHF.22,23 Importantly, these trials did not include patients in CGS. These medications are contraindicated in patients with hypotension in the setting of AMI. Once shock has resolved, ACE-I and/or ARBs can be initiated at low doses, provided no contraindications to these drugs exist.
MECHANICAL COMPLICATIONS
Ventricular Septal Defect
VSD is a serious complication of AMI that increases the risk of mortality substantially, even in patients who have undergone urgent surgical correction. In the prethrombolytic era, its occurrence was between 1% and 2%, and it accounted for approximately 5% of AMI-related deaths. The incidence has decreased with the use of thrombolytic agents, as demonstrated in the GUSTO-I Trial, where this was 0.2%.24 Also noteworthy, in GUSTO-I the most important predictors of VSD were advanced age, female sex, anterior infarct location, and no current smoking.24 In addition, a history of hypertension was common among patients. Angiographically, >50% of the patients had two- and three-vessel coronary disease, and those patients with a VSD were more likely to have had total occlusion of the infarct-related artery.24
VSD typically presents in the first week after MI and usually within 3 to 5 days of symptom onset. It was noted to occur earlier in the GUSTO-I and SHOCK trials, with a median time of 1 day and 16 hours from MI symptom onset, respectively.24,25 Many of the patients in both of these trials received thrombolytic therapy, which has led investigators to hypothesize that timely administration of thrombolytic therapy restores patency of the infarct-related artery, thereby preventing or limiting the extensive transmural necrosis required for ventricular septal rupture. If VSD occurs with lytics, the presentation of the VSD is earlier as the use of lytics might exacerbate the myocardial hemorrhage associated with MI.24
VSD occurs with equal frequency in anterior, inferior, or posterior MIs. When it occurs in the setting of a right coronary artery (RCA) or dominant left circumflex artery (LCx) occlusion, the location of the defect is typically in the posterobasal region of the septum as opposed to the apical–septal region, seen with anterior infarctions. Posterobasal ruptures are often complex, with serpiginous courses containing multiple small defects. This differs from the direct, through-and-through communication sometimes seen with apical–septal defects. Further, posterobasal VSDs are in close proximity to the mitral and tricuspid valves. These anatomic issues make surgical and percutaneous closure of posterobasal ruptures more difficult. Additionally, patients with inferior or posterobasal infarctions often present with varying degrees of RV infarction, which adds to management complexity.
Symptoms and Signs
VSD results in a left-to-right shunt that leads to RV volume overload, increased pulmonary blood flow with reduced systemic blood flow. These hemodynamic alterations often lead to shock. Patients demonstrate signs of biventricular failure that include elevated neck veins and pulmonary edema. Peripheral edema is uncommon in the acute setting, as is displacement of the point of maximal impulse. There is often a loud, holosystolic, precordial murmur that has widespread radiation. A palpable, precordial thrill is present in >50% of these patients.26
Diagnosis
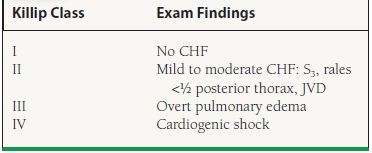
The physical examination is diagnostic in most patients with a VSD. The test of first choice to confirm its presence is TTE with color Doppler imaging. A basal VSD can be visualized in multiple views, including the parasternal long-axis view with medial angulation, the apical long-axis view, the subcostal long-axis view, and the parasternal short-axis view (Fig. 42.1). An apical VSD is best visualized in the apical four-chamber view, although it too can be appreciated in multiple views.
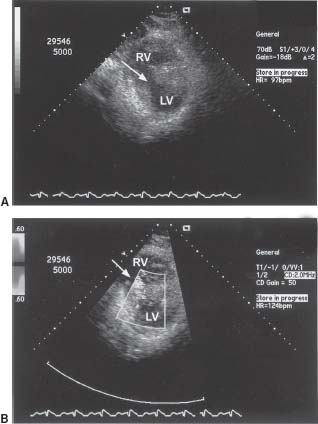
FIGURE 42.1 A: Subcostal short-axis view demonstrating an inferobasal VSD (arrow). B: Same view with Doppler across the defect, demonstrating a left-to-right shunt. RV, right ventricle; LV, left ventricle.
Diagnosis can be confirmed by right heart catheterization with a saturation run. This is performed by sequentially sampling blood from the pulmonary artery (PA), RV, right atrium (RA), superior vena cava (SVC), and inferior vena cava (IVC). At least a 7% step-up in the oxygen saturation from RA to PA is required to diagnose a LV to RV level. Subsequently, by using the O2 saturation values, one can calculate the degree of shunting, which is expressed as the ratio of pulmonary to systemic blood flow (Qp:Qs) (Fig. 42.2).
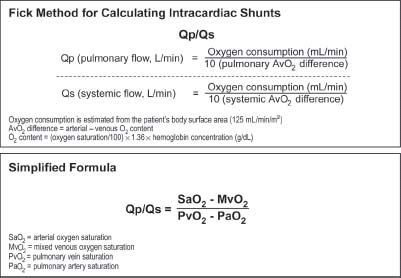
FIGURE 42.2 Calculation of left-to-right intracardiac shunts. When no shunt exists, MvO2 = pulmonary artery saturation. When a left-to-right shunt is present, MvO2 = the saturation in the chamber prior to the oxygen step-up. If there is no right-to-left shunt, and PvO2 is not collected, it is assumed to be 95%.
Lastly, VSD can be diagnosed by ventriculography. Typically, the best view for this is the LAO view with cranial projection, such as LAO 60 degrees with 20 degrees of cranial angulation. This image nicely demonstrates the full length of the septum and, with contrast injection, passage of the contrast material will be seen crossing into the RV (Fig. 42.3).
FIGURE 42.3 A: RAO 20-degree projection demonstrating filling of the left ventricle (LV). B: Same view demonstrating filling of the right ventricle (RV) from the LV through an inferoapical VSD (arrow). C: LAO 40-degree, cranial 25-degree projection demonstrating filling of the LV. D: Same view demonstrating filling of the RV from the LV through an inferoapical VSD (arrow).
Treatment
Emergency cardiac surgery with concomitant CABG is recommended for patients who present with VSD following AMI. This applies to both stable and unstable patients because stability can change rapidly if the necrotic edges of the defect expand abruptly. While surgical consultation is being obtained, patients should be placed in the ICU for invasive hemodynamic monitoring, initial treatment of shock, and consideration of IABP insertion. If the patient is not hypotensive, a short-acting vasodilator such as nitroprusside can be used to optimize hemodynamics.4 Percutaneous closure for a VSD complicating AMI has been reported in small case series.27 It remains investigational, as patient numbers were small, follow-up was short, different devices were used, and some patients underwent closure several weeks following the acute event. This technique however might be used as a last resort in patients who are poor surgical candidates because complete closure is typically not achieved with percutaneous devices.
Even in patients who undergo emergency surgical repair, mortality at 30 days in the thrombolytic era is estimated to be approximately 50%. This is in comparison to a >90% 30-day mortality in patients managed medically.24,25 This underscores the importance of prompt recognition, early placement of an IABP, and expeditious triage to the operating room for surgical repair.
Acute Mitral Regurgitation from Papillary Muscle Rupture or Functional Mitral Regurgitation
MR in the setting of AMI can occur for various reasons. It can result from papillary muscle rupture, which complicates 1% of AMIs and results in approximately 5% of AMI-related deaths. It can also result from abnormal coaptation of the mitral valve leaflets during systole due to dyssynchronous contraction of the LV walls. The latter may be seen in the acute setting when segmental LV wall dysfunction leads to restricted motion of the leaflet leading to MR. In the absence of early revascularization with scar formation, this type of MR might become chronic over time.
Although any degree of MR following AMI has been associated with an increase in mortality, papillary muscle rupture carries the gravest prognosis. In patients who develop severe MR, due to restrictive leaflet motion, the infarct is often extensive. In contrast, papillary muscle rupture is more often the result of a small infarction affecting the papillary muscle itself and typically occurs between the second and seventh days following AMI.26 Rupture of the posteromedial papillary muscle in the setting of an inferior infarction is more common because it has a single vessel blood supply from the posterior descending branch of the dominant coronary artery. The anterolateral papillary muscle receives a dual blood supply from the left anterior descending (LAD) and LCx arteries, making it more resistant to necrosis.
Symptoms and Signs
Severe MR caused by papillary muscle rupture or a restrictive leaflet manifests as sudden shortness of breath followed by rapid hemodynamic deterioration. The median time to its development in the SHOCK Trial registry was 12.8 hours.28 Also noteworthy in the SHOCK Trial Registry was that ST elevation was less frequent in those patients with CGS and severe MR in comparison to patients with CGS caused by severe LV dysfunction without MR.28 This important observation shows that an ECG with a relatively benign appearance in the setting of abrupt and severe hemodynamic deterioration should raise the suspicion of acute MR. Signs of shock are typically present with pulmonary rales. A precordial, systolic murmur may be heard, and its quality varies significantly from patient to patient. In some cases there is no appreciable murmur due to the rapid equilibration of pressures between the left ventricle and left atrium.
Diagnosis
Although physical examination is quite helpful, accurate diagnosis can be made by TTE or transesophageal echocardiography (TEE) with color Doppler imaging. The left ventricular ejection fraction (LVEF) may be normal or even supranormal in the case of papillary muscle rupture, as the infarcts occurring in this setting are often less extensive and the MR possesses an unloading effect on the left ventricle. If rupture has occurred, one will see the head of the papillary muscle attached to a flail mitral leaflet (Fig. 42.4).
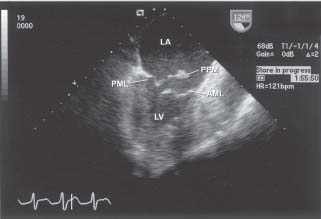
FIGURE 42.4 TEE at 124 degrees at the midesophageal level demonstrating papillary muscle rupture and a flail posterior mitral leaflet. LV, left ventricle; LA, left atrium; PML, posterior mitral leaflet; AML, anterior mitral leaflet; PPM, posterior papillary muscle.
Right heart catheterization (RHC), though important for hemodynamic monitoring and therapy in MR complicating AMI, should not be used as a diagnostic modality. It can, however, help to confirm the diagnosis. In addition to an elevated PCWP, there will often be giant V waves in the PCWP tracing (Fig. 42.5). It should be noted however that large V waves are also seen in patients with acute VSD. Left ventriculography can also confirm the diagnosis, but it is often unnecessary because echocardiography is sufficient to make both diagnostic and treatment decisions.
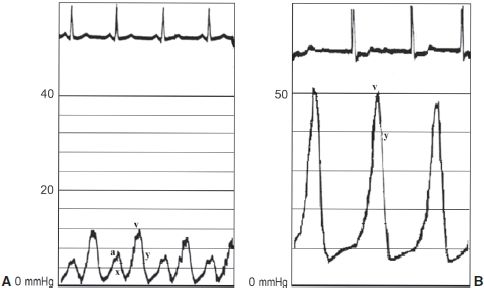
FIGURE 42.5 A: PCWP tracing representing normal waveforms during the cardiac cycle. B: PCWP tracing representing severe MR in a patient in atrial fibrillation. Note the giant V wave that represents regurgitant volume into the left atrium during ventricular systole.



