Complications of Acute Myocardial Infarction
Michael P. Brunner
Venu Menon
I. INTRODUCTION.
In-hospital mortality after acute myocardial infarction (MI) is primarily caused by circulatory failure from severe left ventricular (LV) dysfunction and/or other acute complications of MI. These complications can be broadly classified as mechanical, arrhythmic, embolic, and inflammatory (e.g., pericarditis).
II. MECHANICAL COMPLICATIONS.
Mechanical complications of acute MI include ventricular septal rupture (VSR), acute mitral regurgitation (MR), ventricular free wall rupture, ventricular pseudoaneurysm, and ventricular aneurysm.
A. Ventricular septal rupture
1. Clinical presentation.
VSR occurred in 1% to 2% of patients after acute MI in the prethrombolytic era and accounted for 5% of the periinfarction mortality. The incidence has dramatically decreased in the postthrombolytic era. In the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries 1 (GUSTO-1) trial, the incidence of VSR was approximately 0.2%, occurring with equal frequency in anterior and non-anterior locations. VSR is more likely to occur in patients who are older, female, and hypertensive and have no prior history of smoking. It commonly occurs in the setting of a first MI, in the background of delayed or absent reperfusion therapy. Angiography usually reveals an absence of collateral circulation to the infarct zone. VSR may develop as early as 24 hours after MI but is usually seen 2 to 5 days after MI. Fibrinolytic therapy is not associated with increased risk of VSR but may accelerate rupture in vulnerable subjects, accounting for the “early hazard” observed with treatment over placebo in randomized clinical trials.
a. Signs and symptoms. Patients with post-MI VSR may appear relatively comfortable early in the disease course. Recurrence of angina, pulmonary edema, hypotension, and shock may develop abruptly later in the course. Alternatively, precipitous onset of hemodynamic compromise characterized by hypotension, biventricular failure, and a new murmur may be the initial manifestation.
b. Physical findings. The diagnosis should be suspected when a new pansystolic murmur develops, especially in the setting of worsening hemodynamic profile and biventricular failure. For this reason, it is important that all patients with MI have a well-documented cardiac examination at presentation and frequent evaluations thereafter. This assumes critical importance as systems struggle to achieve optimal door-to-balloon times.
(1) The murmur is usually best heard at the lower left sternal border; it is accompanied by a thrill in 50% of the cases. In patients with a large
VSR and severe heart failure or cardiogenic shock, the murmur may be of low intensity or inaudible, but the absence of a murmur does not rule out VSR.
VSR and severe heart failure or cardiogenic shock, the murmur may be of low intensity or inaudible, but the absence of a murmur does not rule out VSR.
TABLE 3.1 Differential Diagnosis of a New Systolic Murmur after Acute Myocardial Infarction | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
(2) Several features differentiate the murmur of VSR from that of acute MR (Table 3.1). The murmur may radiate to the base and the apex of the heart. A third heart sound (S3), loud P2, and signs of tricuspid regurgitation may be present.
2. Histopathology.
The defect usually occurs at the myocardial infarct border zone, located in the apical septum with anterior MI and in the basal posterior septum with inferior MI. A VSR almost always occurs in the setting of a transmural MI. The defect may not always be a single large defect; a meshwork of serpiginous channels can be identified in 30% to 40% of patients. Multiple fenestrations are especially common with inferior MIs.
3. Diagnostic testing
a. An electrocardiogram (ECG) may show atrioventricular (AV) node or infranodal conduction abnormalities in approximately 40% of patients.
b. Echocardiography
(1) Transthoracic echocardiography is the test of choice for the diagnosis of VSR. It is important for the clinician to interrogate the area of interest with color Doppler ultrasound. Lowering the Nyquist limit will enable definition and help define the size of the defect. The echocardiogram will also provide insight into the feasibility of utilizing temporizing percutaneous closure devices in this setting.
(a) Basal VSR is best visualized in the parasternal long axis with medial angulation, the apical long axis, and the subcostal long axis.
(b) Apical VSR is best visualized in the apical four-chamber view.
(2) In some cases, transesophageal echocardiography may help in determining the extent of the defect and assessing suitability for potential percutaneous closure.
(3) Echocardiography may help determine the size of the defect and the magnitude of the left-to-right shunt by comparing flow across the pulmonary valve with flow across the aortic valve.
(4) An assessment of right ventricular (RV) and LV function is key to prognostication and management as they remain important determinants of mortality.
c. Right heart catheterization. Pulmonary artery (PA) catheterization with oximetry measurement can help diagnose VSR by demonstrating an oxygen saturation step-up in the RV and PA. The location of the increase is significant because there have been case reports of enhanced oxygen saturation in the peripheral PA due to acute MR. Diagnosis involves fluoroscopically guided measurement of the oxygen saturation in the superior and inferior venae cavae; high, mid, and low right atrium (RA); base, mid, and apical levels of the RV; and the PA.
(1) Normal oxygen saturations for these chambers are 64% to 66% in the superior vena cava (SVC), 69% to 71% in the inferior vena cava (IVC), 64% to 67% in the RA, 64% to 67% in the RV, and 64% to 67% in the PA.
(2) An oxygen step-up at the level of the RV is characteristically seen with VSR. A left-to-right shunt across the ventricular septum typically results in a 5% or greater increase in oxygen saturation between the RA and the RV or PA.
(3) Shunt fraction is calculated as follows:
In this equation, Qp is pulmonary flow; Qs is systemic flow; SaO2 is peripheral arterial oxygen saturation; MvO2 is mixed venous oxygen saturation; PvO2 is pulmonary venous oxygen saturation; and Pao2 is pulmonary arterial oxygen saturation. Mvo2 is calculated by multiplying the SVC oxygen saturation by three, adding the IVC oxygen saturation, and then dividing the sum by four. Pvo2 is generally assumed to be equal to the peripheral oxygen saturation. Qp/Qs ≥ 2 suggests the presence of a considerable shunt. In the acute MI setting, any VSR should be considered for urgent surgical repair, regardless of the shunt fraction.
(4) For a patient with an intracardiac shunt, cardiac output measured by means of the thermodilution technique is inaccurate; the Fick method should be used. The key to measurement of accurate systemic flow in the presence of a shunt is that the oxygen content measured in the PA will be abnormally elevated and must be measured in the chamber immediately proximal to the shunt (i.e., the RA or the SVC and IVC in the case of VSR). The Fick equation is normally calculated as follows:
d. Left heart catheterization. Ventriculography performed after angiography or percutaneous intervention (PCI) may reveal VSR if the suspicion is high. Visualization is best in the left anterior oblique projection with cranial angulation.
e. Cardiac MRI and CT are additional imaging modalities that can be utilized. However, the studies are more difficult to perform in hemodynamically unstable patients and do not play a significant role in this setting.
4. Therapy
a. Priority of therapy. Urgent surgical closure is the treatment of choice (AHA/ACC class I recommendation), especially when the patient’s condition is stable because hemodynamic deterioration in this setting is unpredictable. Although initial reports suggested that delaying surgery to allow healing of friable tissue improved surgical mortality, it was likely that lower mortality
was simply a result of selection bias. The mortality rate for patients with VSR treated medically is 24% at 72 hours and 75% at 3 weeks.
was simply a result of selection bias. The mortality rate for patients with VSR treated medically is 24% at 72 hours and 75% at 3 weeks.
b. Vasodilators can decrease left-to-right shunt and increase systemic flow by means of reducing systemic vascular resistance (SVR); however, a greater decrease in pulmonary vascular resistance may actually increase shunting. The vasodilator of choice is intravenous nitroprusside, which is started at 0.5 to 0.8 µg/kg/min and titrated to a mean arterial pressure (MAP) of 70 to 80 mm Hg.
c. An intraaortic balloon pump (IABP) should be inserted as early as possible as a bridge to a surgical procedure, unless there is marked aortic regurgitation. IABP counterpulsation decreases SVR, decreases shunt fraction, increases coronary perfusion, and maintains blood pressure. After insertion of an IABP, vasodilators can be tailored with hemodynamic monitoring.
d. Surgical therapy
(1) Cardiogenic shock and multisystem failure are associated with high surgical mortality, further supporting earlier operations on these patients before complications develop. Mortality in patients with cardiogenic shock and VSR was 81% in the SHOCK (SHould we emergently revascularize Occluded coronaries for Cardiogenic shocK?) trial registry (1).
(2) Surgical mortality is high among patients with basal septal rupture associated with inferior MI (70% compared with 30% in patients with anterior infarcts) because of the greater technical difficulty and the need for concomitant mitral valve repair in these patients, who often have coexisting MR. RV dysfunction due to infarction and/or pressure and volume overload further increases the risk profile of these subjects.
e. Percutaneous therapy. Although surgical closure remains the treatment of choice for VSR, emerging data suggest that percutaneous closure may be a viable treatment for high-risk surgical patients and patients in whom surgical closure has failed. In a series of 29 patients treated with percutaneous VSR closure at a median time of 1 day after MI, mortality at 30 days in patients treated with successful device placement was 36% and 86% without and with cardiogenic shock, respectively. At a median follow-up of 730 days, mortality was 36% and 93%, respectively. In our institution, a percutaneous approach is utilized for temporary palliation and as a bridge to surgical repair only in patients considered too high risk to undergo surgery.
B. Acute MR.
The incidence of acute MR after MI was 13% to 39% in large registries such as GUSTO-1 and SHOCK. Fibrinolytic agents decrease the overall incidence, but rupture may occur earlier in the post-MI period. MR, even if clinically silent, is a predictor of poor prognosis in MI. Multiple mechanisms may account for acute MR. These include dilation of the mitral valve annulus as a result of LV dilation; papillary muscle dysfunction with a concomitant ischemic regional wall motion abnormality near the insertion of the posterior papillary muscle; and partial or complete rupture of the chordae or papillary muscle. Severe MR caused by papillary muscle rupture is a life-threatening complication of acute MI. Historical reports indicate that papillary muscle rupture occurs between days 2 and 7. However, the SHOCK registry revealed a median time to papillary muscle rupture of 13 hours. Acute severe MR accounted for 7% of the cases of cardiogenic shock and 5% of mortality after acute MI in the SHOCK registry.
1. Clinical presentation
a. Signs and symptoms. These are variable and depend on the anatomy of the papillary muscle involved, the mechanism of valvular dysfunction, and the extent of injury. Complete transection of the papillary muscle is rare and usually results in immediate cardiogenic shock and death. Patients with partial or complete rupture of one or more heads of the papillary muscle lose
significant leaflet support. The resultant torrential MR can result in pulmonary edema and severe respiratory distress.
significant leaflet support. The resultant torrential MR can result in pulmonary edema and severe respiratory distress.
b. Physical findings. A new pansystolic murmur that is audible at the cardiac apex with radiation to the axilla or the base of the heart suggests acute MR. In posterior papillary muscle rupture, the murmur radiates to the left sternal border and may be confused with the murmur of VSR or aortic stenosis. The intensity of the murmur does not predict the severity of the MR. The murmur may often be quiet, soft, or absent in patients with poor cardiac output or in persons with elevated left atrial pressure due to the rapid equilibration of pressures. Resting tachycardia and mechanical ventilation can also make murmur recognition challenging.
2. Pathophysiology.
Papillary muscle rupture is more common with an inferior MI because the posteromedial papillary muscle receives blood supply from the posterior descending artery, whereas the anterolateral papillary muscle has dual blood supply from the left anterior descending (LAD) and circumflex arteries. Papillary muscle rupture is more likely to occur in patients with a first MI, and in many patients the infarct size may be relatively small. The discordance between the degree of hemodynamic instability and the extent of myocardium in jeopardy is often a clue to the underlying condition.
3. Diagnostic testing
a. An ECG usually shows evidence of recent inferior or posterior MI.
b. A chest radiograph may demonstrate pulmonary edema. In some patients, focal pulmonary edema may be seen in the right upper lobe because of flow directed at the right pulmonary veins.
c. Transthoracic echocardiography with Doppler and color flow imaging is the diagnostic modality of choice.
(1) The mitral valve leaflet is usually flail with severe MR.
(2) Color Doppler imaging is useful in differentiating papillary muscle rupture with severe MR from VSR after MI.
d. Transesophageal echocardiography. Transthoracic echo may underestimate the degree of acute MR. Rapid equalization of pressure, resting tachycardia, and poor acoustic windows may contribute to this finding. An eccentric jet in this setting should lead to the performance of transesophageal echocardiography to quantify the severity and elucidate the mechanism of MR.
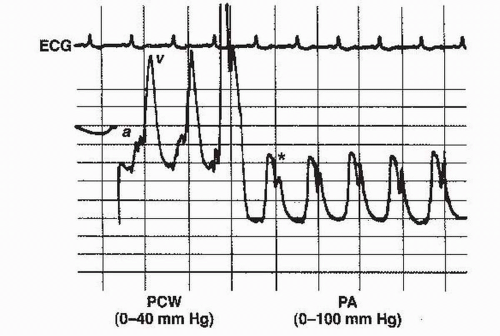
e. PA catheterization. Hemodynamic monitoring with a PA catheter may reveal large V waves in the pulmonary capillary wedge pressure (PCWP) tracing. However, patients with VSR may also have large V waves because of increased pulmonary venous return in a normal-sized and normally compliant left atrium. Among patients with severe MR and reflected V waves in the PA tracing, oxygen saturation in the PA may be higher than that in the RA, complicating differentiation from VSR. There are two methods for differentiating MR from VSR:
(1) Prominent V waves in the PA tracing before the incisura are almost always associated with acute severe MR (Fig. 3.1).
(2) Blood for oximetry is obtained with fluoroscopy to ensure sampling from the main PA rather than distal branches.
4. Therapy
a. Priority of therapy. Papillary muscle rupture should be identified early. Patients should receive aggressive medical therapy and consideration for emergent surgical repair.
b. Vasodilator therapy is beneficial in the treatment of patients with acute MR. Intravenous nitroprusside decreases SVR, reduces regurgitant fraction, and increases stroke volume and cardiac output. Nitroprusside is started at 0.5 to 0.8 µg/kg/min and is titrated to an MAP of 70 to 80 mm Hg.
c. Intraaortic balloon pump. Vasodilator therapy is contraindicated in patients with significant hypotension and an IABP should be inserted promptly. An IABP decreases LV afterload, improves coronary perfusion, and increases forward cardiac output. Patients with hypotension can often be given vasodilators after insertion of an IABP to improve hemodynamic values.
d. Percutaneous therapy. Improvement in hemodynamic values and reduction in MR has been reported after PCI in patients with severe MR caused by papillary muscle ischemia rather than rupture. However, this is a relatively rare clinical presentation. PCIs have no role in true papillary muscle rupture.
e. Surgical therapy should be considered immediately for patients with papillary muscle rupture.
(1) The prognosis is very poor among patients treated medically. Even though perioperative mortality (20% to 25%) is higher than that for elective surgical treatment, surgical therapy should be considered for every patient.
(2) Coronary angiography should be performed before surgical correction, because revascularization is associated with improved short- and longterm mortality.
C. Ventricular free wall rupture
1. Clinical presentation.
The incidence of ventricular free wall rupture after MI in the reperfusion era is < 1%. However, ventricular free wall rupture accounts for approximately 10% of mortality after MI. In the SHOCK registry, in-hospital mortality associated with ventricular rupture was > 60%. Rupture occurs in the
first 5 days in 50% of patients and within 2 weeks in 90% of patients. Ventricular free wall rupture occurs in the setting of a transmural MI. Risk factors include advanced age, female sex, first MI, and poor coronary collateral vessels.
first 5 days in 50% of patients and within 2 weeks in 90% of patients. Ventricular free wall rupture occurs in the setting of a transmural MI. Risk factors include advanced age, female sex, first MI, and poor coronary collateral vessels.
a. Signs and symptoms
(1) Acute course. With acute rupture, patients develop tamponade, electromechanical dissociation, and sudden death. Sudden onset of chest pain with straining or coughing may suggest the onset of myocardial rupture.
(2) Subacute course. Some patients may have a contained rupture and present subacutely with pain suggestive of pericarditis, nausea, and hypotension. In a large retrospective analysis of post-MI patients, 2.6% of patients were found to have sustained subacute ventricular free wall rupture. Immediate bedside echocardiography may reveal localized pericardial effusion or pseudoaneurysm.
b. Physical findings.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree