Complications
Conor Barrett
Robert Schweikert
Walid Saliba
Jennifer Cummings
J. David Burkhardt
Oussama Wazni
Andrea Natale
All invasive procedures carry associated risks of complications. This is the case with all currently available strategies for catheter ablation of atrial fibrillation (AF). Different institutions cite differing rates of such complications; this is probably a reflection of many variables including varying degrees of expertise and training, case volumes and complexities, as well as differing procedural methods. Therefore, in this chapter the complication rates quoted are a reflection of published experience. However, it is important to have a realistic awareness of complications and complication rates in one’s own center. A discussion of benefits and potential risks obviously forms the basis for informed consent for all procedures, not the least AF ablation.
Given the nature of AF ablation, overall complication rates are relatively low and some (e.g., pulmonary vein [PV] stenosis), which were once not infrequent, are much less commonly seen as the procedure has developed over time. Other complications (e.g., atrial-esophageal fistula), although rarely encountered have gained special attention due to their associated high morbidity and mortality rates. An overall major complication rate of 5.9% was reported in a worldwide comprehensive survey of AF ablation (1). It may be that complication rates may have diminished since this study was published as understanding of AF mechanisms and ablation techniques have improved. This chapter focuses on the more-commonly encountered complications and gives potential strategies for their avoidance, as well as management suggestions if they are to occur.
Background
Access-related complications, as in most percutaneous procedures, are the most commonly encountered. These range from simple hematomas to fistulas, pseudoaneurysms, and retroperitoneal hemorrhage. There are certain patient-specific factors that can predispose to an increased incidence of access-related complications. Advancing age and obesity in particular are associated with an increased rate of access-related complications. Venous access, particularly in the obese patient, may be troublesome due to unclear anatomical landmarks and the relative depth of the femoral veins. The incidence of
femoral pseudoaneurysm or arteriovenous fistula is reported to be about 0.5% (1). Many centers now perform AF ablations on patients who have a therapeutic INR at the time of the procedure, or “bridge” them with heparin products after the procedure, while awaiting INR levels to become therapeutic. The procedure also obviously necessitates heparin infusion. All of these predispose to hematoma formation at the site of access.
femoral pseudoaneurysm or arteriovenous fistula is reported to be about 0.5% (1). Many centers now perform AF ablations on patients who have a therapeutic INR at the time of the procedure, or “bridge” them with heparin products after the procedure, while awaiting INR levels to become therapeutic. The procedure also obviously necessitates heparin infusion. All of these predispose to hematoma formation at the site of access.
Prevention
We have found that direct visualization of the internal jugular vein facilitates successful cannulation of the vein without inadvertent damage to nearby structures, most notably the carotid artery. This may be aided by advancing a long 0.035 wire or a deflectable catheter from the femoral vein into the vein and puncturing the vein under x-ray guidance. Another method, avoiding the use of x-ray, is by using ultrasound guidance (Site-Rite; Bard Access Systems, Salt Lake City, UT). We have found this to be an excellent way to directly visualize the internal jugular vein at the time of cannulation, therefore avoiding collateral damage to the medially located carotid artery and laterally located sternocleidomastoid muscle. This approach also allows access to be gained higher in the neck, minimizing the risk of lung puncture.
Femoral venous access is achieved caudal to the inguinal ligament. In achieving femoral venous access in more obese individuals, ultrasound can sometimes assist. Even if it is not possible to assess the location of the femoral vein under ultrasound, the pulsatile femoral artery is almost always apparent. This landmark then guides venous access and avoids inadvertent damage to the artery. In order to reduce the risk of fistula formation, it is important not to redirect the access needle once it is advanced below the skin level.
Even in the presence of a clean venous access, vigorous manipulation of the transseptal sheaths can lead to bleeding around the access sites and should be avoided.
Diagnosis and Management
If they do occur, most hematomas can be managed conservatively with manual pressure. Thrombin injection into the neck of pseudoaneurysms may be performed, resulting in resolution of this complication. Rarely, it is necessary for pseudoaneurysms and fistulas to undergo surgical repair if more conservative methods fail. Retroperitoneal bleeding, suggested by back and flank pain, necessitates cessation of the procedure and reversal of anticoagulation in the first instance. Surgical advice should be sought early as retroperitoneal bleeding may not cease without intervention. The diagnosis of retroperitoneal bleeding is confirmed by contrast-enhanced computerized tomography (CT).
Background
The incidence of PV stenosis has deceased significantly since the initial strategies of targeting foci within the PVs themselves. In fact, the observation of such a complication with earlier ablation strategies was one of the main reasons for the alteration of strategies, moving lesion sets away from the PVs themselves and into the left atrium (LA). The left-sided veins, particularly the inferior PV (LIPV), appear to be the most vulnerable to stenosis (2). Figure 22.1 depicts a moderate stenosis of the LIPV in an asymptomatic patient.
Differing rates of PV stenosis are reported. This probably reflects differing ablation techniques and follow-up of patients, as even severe PV stenosis may be asymptomatic.
In the worldwide survey of AF, severe PV stenosis was reported in 1.6% of patients (1). In one study comparing different AF ablation strategies in 608 patients, different rates of severe PV stenosis were seen depending on the ablation strategy utilized (3). When based on an electroanatomic approach, PV stenosis was observed in 15.5%; with a circular mapping with distal isolation technique, PV stenosis was observed in 20%; and with a circular mapping with ostial isolation based on PV angiography, stenosis was observed in 2.9%. The lowest rates of PV stenosis were seen using a circular mapping catheter guided by intracardiac echocardiography (ICE) (in 1.45%) and, when energy delivery based on visualization of microbubbles was employed, no severe PV stenosis was observed. An overall stenosis rate of 5% was seen. Figure 22.2 demonstrates a shower of microbubbles while RF energy is being delivered near the right superior PV (RSPV). Another study identified moderate or severe stenosis in 3.8% of PVs isolated using an electroanatomic approach (4). PV occlusion (defined as >95% stenosis) was seen in 16 of 1,780 patients (0.8%) followed at the Cleveland Clinic (5).
In the worldwide survey of AF, severe PV stenosis was reported in 1.6% of patients (1). In one study comparing different AF ablation strategies in 608 patients, different rates of severe PV stenosis were seen depending on the ablation strategy utilized (3). When based on an electroanatomic approach, PV stenosis was observed in 15.5%; with a circular mapping with distal isolation technique, PV stenosis was observed in 20%; and with a circular mapping with ostial isolation based on PV angiography, stenosis was observed in 2.9%. The lowest rates of PV stenosis were seen using a circular mapping catheter guided by intracardiac echocardiography (ICE) (in 1.45%) and, when energy delivery based on visualization of microbubbles was employed, no severe PV stenosis was observed. An overall stenosis rate of 5% was seen. Figure 22.2 demonstrates a shower of microbubbles while RF energy is being delivered near the right superior PV (RSPV). Another study identified moderate or severe stenosis in 3.8% of PVs isolated using an electroanatomic approach (4). PV occlusion (defined as >95% stenosis) was seen in 16 of 1,780 patients (0.8%) followed at the Cleveland Clinic (5).
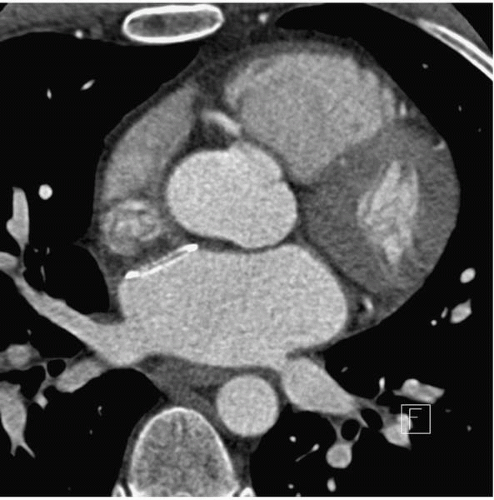 Figure 22.1. An axial CT image of a moderate stenosis of the left inferior pulmonary vein, seen 3 months after AF ablation. The patient was asymptomatic |
In a canine model, it has been shown that the mechanisms of PV stenosis after radiofrequency (RF) ablation include fibrocellular intimal proliferation, endoluminal thrombus formation, endocardial contraction, and proliferation of elastic lamina (6). Proliferation of the intima was observed in all stenotic PVs in this model and was responsible for much of the narrowing. Further histopathologic analysis was derived from a patient who had undergone two AF ablation procedures and developed PV stenosis in all four veins, eventually requiring lobectomy after previously undergoing patch annuloplasty and subsequent angioplasties (7). Histopathologic examination revealed marked medial thickening and intimal hyperplasia of large and small PVs and arteries, as well as focal organizing thrombi in the small arteries. The lung tissue showed changes consistent with extensive prior hemorrhage. The authors observed that chronic PV stenosis resulted in irreversible venous and arterial morphologic changes throughout the lung. These were seen not only at the regions targeted for ablation but also in the vasculature distant from the site of ablation. It was therefore postulated, as significant pathologic changes were seen distant from the proximal PVs, that stenting or
angioplasty of the proximal veins may not result in the abolition of the development of subsequent symptomatic pulmonary hypertension (7). This may, however, be a reflection on the severity and quantity of veins affected. Delays in identification and treatment of severe PV stenosis may therefore lead to changes in the more distal pulmonary vasculature, which is not reversible after treatment of the proximal PV stenosis. It has, however, been demonstrated that earlier identification and treatment results in improvement of symptoms and pulmonary pressures in most patients with PV stenosis.
angioplasty of the proximal veins may not result in the abolition of the development of subsequent symptomatic pulmonary hypertension (7). This may, however, be a reflection on the severity and quantity of veins affected. Delays in identification and treatment of severe PV stenosis may therefore lead to changes in the more distal pulmonary vasculature, which is not reversible after treatment of the proximal PV stenosis. It has, however, been demonstrated that earlier identification and treatment results in improvement of symptoms and pulmonary pressures in most patients with PV stenosis.
Prevention
Regardless of the ablation strategy employed, avoiding delivery of RF lesions within the PVs themselves is the most important precaution to minimize the risk of this complication. Many now advocate ablating well away from the PV ostia to help minimize this complication. Care must be taken when relying on fluoroscopy and mapping systems to ensure that the true ostia of the PVs are identified correctly. Ablation distal to these points should be avoided at all costs.
Real-time imaging with ICE aids in the definition of the true PV ostia and allows for monitoring of catheter migration into the veins during lesion delivery. Furthermore, monitoring of catheter position using a 3-D navigational system aids in identifying inappropriate catheter positioning. The delivery of excess RF energy near the PVs also contributes to PV stenosis, thus power and duration of lesion delivery need to be balanced. Power titration may be guided by microbubble formation if a solid-tip ablation catheter is employed, resulting in a much lower complication rate (Fig. 22.2) (3). In a series from Bordeaux looking at acute PV stenosis at the end of the ablation procedure, a low rate
of immediate PV stenosis (0.7%) was seen when using an irrigated-tip catheter (8). This was attributed to lesion delivery as proximal (close to the atrium) as possible, limiting power to 25 W for the LIPV (which is the most vulnerable to PV stenosis) and 30 W for the other veins, and to the utilization of a circumferential PV mapping catheter.
of immediate PV stenosis (0.7%) was seen when using an irrigated-tip catheter (8). This was attributed to lesion delivery as proximal (close to the atrium) as possible, limiting power to 25 W for the LIPV (which is the most vulnerable to PV stenosis) and 30 W for the other veins, and to the utilization of a circumferential PV mapping catheter.
Presentation
The pattern of presentation varies greatly, even in the presence of severe PV stenosis. In one study, in 18 patients with severe (>70%) PV stenosis, eight (44%) were asymptomatic, eight (44%) reported dyspnea, seven (39%) reported cough, and five (28%) reported hemoptysis (9). Interestingly, radiological abnormalities were present in 50%. However, the underlying diagnosis was initially missed in seven (39%), four patients being diagnosed with pneumonia, two diagnosed with pulmonary embolism, and one patient was misdiagnosed as having a lung neoplasm. Unfortunately, some patients received inappropriate therapy as a consequence. In another study of 23 patients with symptomatic PV stenosis (at a mean of 100 days after ablation), 19 (82%) reported dyspnea, nine (39%) reported cough, six (26%) reported chest pain, and three (13%) reported hemoptysis (10). Three patients in this study had also been misdiagnosed with bronchitis. In all but one of the patients, physical examination was normal. Others have observed a mean time from ablation to onset of respiratory symptoms to be about 7 weeks (11).
It has been shown that development of symptoms correlates with involvement of more than one PV with severe stenosis, whereas patients with mild and moderate stenosis are asymptomatic (3). Also, in patients with a single PV occlusion, most are asymptomatic if there is no concurrent ipsilateral PV stenosis evident.
Perfusion/ventilation mismatches in patients with severe PV stenosis can be seen, which may lead erroneously to a diagnosis of pulmonary embolism in some patients. Computed tomography (CT) or magnetic resonance imaging (MRI) is the most definitive method to diagnose PV stenosis. An example of PV stenosis as detected by CT is shown in Figure 22.1.
Management
Perhaps more is known about the management and longer-term outcomes of patients with congenital PV stenosis than those with the iatrogenic form. Management of the former has included surgical excision of the stenotic region, reimplantation of the PVs, balloon angioplasty and stenting. Balloon angioplasty in congenital PV stenosis has not proven to be as efficacious as hoped, with high restenosis and recurrent pulmonary hypertension rates (12).
Patients with acquired PV stenosis in all four veins following AF ablation have been treated with balloon angioplasty, with improvement in symptoms and pulmonary pressures (13). Others have reported spontaneous improvement in symptoms in patients with severe PV stenosis, before intervention was undertaken (9). Lung perfusion scans before and after intervention in patients undergoing PV dilatation have shown a significant improvement in perfusion after dilation with or without stenting. Of patients undergoing dilatation, 57% reported symptomatic improvement at 6 months; however severe restenosis was identified in 33%, half of whom had in-stent restenosis (9). This reported experience is in keeping with other data in patients with symptomatic severe PV stenosis. In a study of 23 patients with 34 severe PV stenoses, 20 veins were treated with balloon dilatation alone, whereas 14 veins were stented with 10-mm-diameter bare-metal stents. An immediate symptomatic response was seen; however, over 17 months of follow-up, 14 patients developed in-stent or in-segment
restenosis, requiring repeated interventions in 13 (10). Previous reports have also documented acute success with balloon angioplasty with similar observations of restenosis. A series of 17 patients from the Cleveland Clinic demonstrated improved blood flow to affected lung segments postangioplasty. During follow-up, a repeat dilatation was needed in eight of the 17 patients. Despite this reintervention rate, the vast majority (15 of 17) were asymptomatic at a median of 43 weeks’ follow-up (11).
restenosis, requiring repeated interventions in 13 (10). Previous reports have also documented acute success with balloon angioplasty with similar observations of restenosis. A series of 17 patients from the Cleveland Clinic demonstrated improved blood flow to affected lung segments postangioplasty. During follow-up, a repeat dilatation was needed in eight of the 17 patients. Despite this reintervention rate, the vast majority (15 of 17) were asymptomatic at a median of 43 weeks’ follow-up (11).
Thus, a high restenosis rate is seen with balloon angioplasty or stenting procedures for acquired PV stenosis. Nonetheless, most advocate dilatation, with or without stenting, of the affected veins(s) if the patient has severe PV stenosis. On the other hand, it remains unclear whether the use of medicated stents would reduce the restenosis rate, as such coated stents in the sizes required for the PVs are not yet available.
In patients who have asymptomatic severe stenosis of a single vein, it is uncertain if these should be treated invasively. Such patients should at least be followed closely to assess for the development of symptoms. Furthermore, although improvement of PV stenosis has been reported, progression to complete PV occlusion has also been seen. Therefore, occurrence of suggestive symptoms in a patient who previously had been shown to have moderate stenosis should prompt reinvestigation for progression of this entity. It has also been noted that late dilatation of completely occluded PVs is not associated with normalization of the ventilation/perfusion mismatch (5). This suggests that earlier intervention, even in the absence of symptoms may be appropriate.
Anticoagulation should be maintained in patients with severe stenosis to prevent thrombotic occlusion of the vein. Given the high rate of restenosis following balloon angioplasty or stenting, it is appropriate to continue oral anticoagulation in this group also.
Background
Atrial-esophageal fistula (AEF) after catheter ablation of AF has gained much attention in recent years and is one of the most-feared potential complications. The initial reports demonstrated a mortality rate of nearly 100% (14,15,16). A prevalence of 0.05% was seen after combining data from two experienced centers (15). This may, however, be an underestimation of the incidence of this complication. The fact that patients present approximately 2 to 4 weeks after the ablation procedure, frequently to other health care providers who are unaware of this potential complication, can lead to a delay in diagnosis and treatment. The nature of the complication and the delay in presentation and diagnosis all conspire to make this a devastating, albeit rare, complication. Atrial-esophageal fistulas are probably the result of thermal injury to the atrium and adjacent esophagus, leading to tissue necrosis and hence fistula formation from the posterior LA to the esophagus.
Prevention
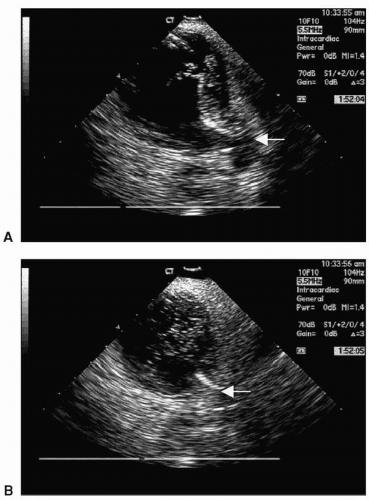
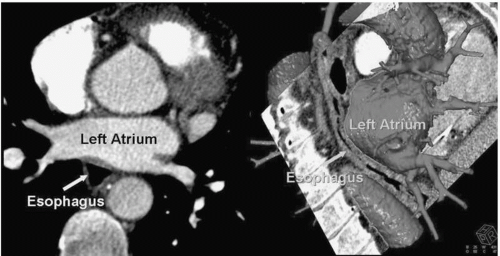
The esophagus is a “moving target best avoided,” lying posterior to the LA. Figure 22.3 gives a representation of the real-time proximity of the LA to the esophagus and Figure 22.4 demonstrates a CT reconstruction showing the relationships of the LA and esophagus.
During ablation of the posterior LA, significant external pressure can be placed on the esophagus, as can be seen in Figure 22.5. Due to its high mortality rate,
incorporation of a strategy aimed at preventing esophageal injury is mandatory during ablation of AF. Necropsy studies have shown that the esophageal course along the posterior LA is variable (17). It was observed that in 40% of cases it coursed through the mid-posterior wall between the right and left PVs, in 40% it was seen to have a leftward course, and in 20% the esophagus was seen closer to the right-sided veins.
The esophageal wall was less than 5 mm from the endocardium in 40% of specimens studied. It is also observed that the posterior LA wall does not have a uniform thickness, being thickest adjacent to the coronary sinus and thinnest more superiorly (17). Another study in 81 patients undergoing AF ablation similarly demonstrated a variable esophageal position, coursing near the right PVs in 23 (28%), left PVs in 31 (38%), and mid-posterior wall in 27 (33%) (18). Interestingly, the mean distance between the endocardial surface of the LA and midesophagus lumen on CT was 4.4 ± 1.2 mm at the area of closest contact, which correlated with real-time imaging at the time of the procedure using ICE (4.2 ± 2.1 mm). Additionally, the thickness of the LA posterior wall, as measured by ICE, was only 2.8 ± 0.9 mm.
incorporation of a strategy aimed at preventing esophageal injury is mandatory during ablation of AF. Necropsy studies have shown that the esophageal course along the posterior LA is variable (17). It was observed that in 40% of cases it coursed through the mid-posterior wall between the right and left PVs, in 40% it was seen to have a leftward course, and in 20% the esophagus was seen closer to the right-sided veins.
The esophageal wall was less than 5 mm from the endocardium in 40% of specimens studied. It is also observed that the posterior LA wall does not have a uniform thickness, being thickest adjacent to the coronary sinus and thinnest more superiorly (17). Another study in 81 patients undergoing AF ablation similarly demonstrated a variable esophageal position, coursing near the right PVs in 23 (28%), left PVs in 31 (38%), and mid-posterior wall in 27 (33%) (18). Interestingly, the mean distance between the endocardial surface of the LA and midesophagus lumen on CT was 4.4 ± 1.2 mm at the area of closest contact, which correlated with real-time imaging at the time of the procedure using ICE (4.2 ± 2.1 mm). Additionally, the thickness of the LA posterior wall, as measured by ICE, was only 2.8 ± 0.9 mm.
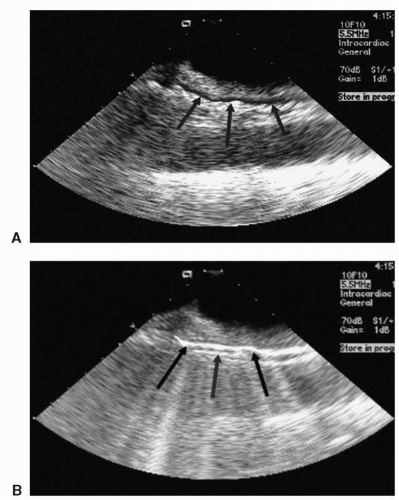
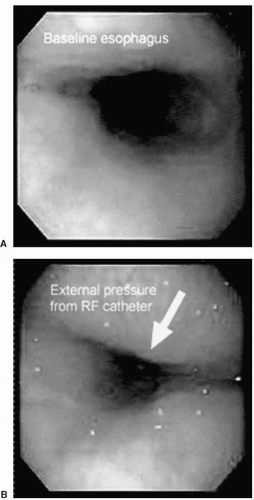 Figure 22.5. Esophagoscopy images at baseline and during RF ablation of the posterior LA. It is apparent that even minimal pressure placed on the esophagus externally from the ablation catheter reduces the esophageal lumen. (Reprinted with permission from Cummings JE, Schweikert RA, Saliba WI, et al. Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the left atrium. Circulation. 2005;112;459-464.) See color insert 2. |
Thus, the inconsistent course of the esophagus, relative to the LA, and the variable distance from LA endocardium to the esophagus pose significant challenges during ablation along the posterior LA. Therefore, knowledge of the esophageal position in each individual is vital. If a cardiac CT is performed prior to the procedure, assessment of the position of the esophagus can be appreciated at that point in time. However, as has been elegantly demonstrated, the esophagus can move substantially even during the ablation procedure, such that the perceived location according to the preprocedure CT may be rendered invalid (19). It is possible to label the esophageal position using a nonfluoroscopic 3-D imaging system (e.g., ESI/NavX, CARTO), which can aid during the procedure. Another method utilized to image the esophagus involves ingestion of radio-opaque contrast to allow real-time visualization during fluoroscopy; there is, however, the risk of aspiration of the contrast if the patient’s trachea is not intubated. ICE is also an excellent way to assess the proximity of the posterior LA to the esophagus. An appreciation of the real-time position of the esophagus can also be aided by monitoring the position of an esophageal thermistor probe during fluoroscopy.
Apart from having an awareness of the esophageal location, titration of delivered power and duration of lesion delivery have also been shown to be important in limiting markers of esophageal damage. This can be achieved by empirically limiting power during posterior LA ablation or, more safely, by titration with ICE guidance. After an index case of atrial esophageal fistula (AEF), one group retrospectively studied ICEvisualized morphological changes in the posterior LA adjacent to the esophageal wall (20). Review of data revealed significant changes in the LA tissue lying anterior to the esophagus. An 8-mm-tip electrode with power of up to 70 W at a maximum of 50°C to 52°C for 60 seconds’ duration or an internally irrigated catheter with power up to 50 W at a maximum of 40°C for 60 seconds’ duration had been used in cases prior to the index AEF case. After the index case, RF power was reduced at areas adjacent to the esophagus, such that 30 to 50 W at 50°C was used for the 8-mm-tip catheter, or 40 W at a maximum of 38°C for the internally irrigated catheter. The duration of RF lesion delivery was titrated (for 10-30 seconds) according to ICE monitoring of microbubble formation or early echogenic lesion formation. PV isolation was still achieved in all patients with such ICE-guided titration without a falloff in efficacy.
Such an ICE-guided strategy is further supported by another study in which patients undergoing AF ablation with an 8-mm-tip catheter had their esophagus course evaluated by CT, 3-D imaging (NavX), or ICE (18). Intraluminal esophageal temperatures were monitored using a thermistor probe. For each lesion, the power, catheter and esophagus temperature, location, and presence of microbubbles were recorded. Power was titrated such that initial delivery was 25 W and 55°C. In the absence of microbubbles, power was titrated upward in 5-W increments every 5 seconds to a maximum of 70 W. If microbubbles were observed, power was titrated downward. If a brisk shower of bubbles was seen, the power was withdrawn and the catheter moved. Each lesion was delivered for 30 seconds. Lesions delivered that were associated with microbubble formation had higher esophageal temperatures than those without (39.3 ± 1.5°C vs. 38.5 ± 0.9°C). Interestingly, there was no correlation between delivered power and esophageal temperatures, which implies that monitoring is important during ablation of the posterior LA. The esophageal thermistor has been found to be a good aid as such. It is important that this is moved to follow the ablation catheter position during lesion delivery on the LA posterior wall to give more-accurate information on the change of local esophageal temperature. As was observed in the study by Cummings et al. (16), even a small elevation in esophageal temperature can be associated in tissue overheating and microbubble formation, and
should prompt the ablation catheter to be repositioned and downward titration or withdrawal of RF energy. Esophageal temperatures have been observed to increase, even after cessation of RF delivery. Also, when using the thermistor probe as a guide, it is important to remember that the absence of a temperature rise may give a false sense of security if it has not been adequately positioned.
should prompt the ablation catheter to be repositioned and downward titration or withdrawal of RF energy. Esophageal temperatures have been observed to increase, even after cessation of RF delivery. Also, when using the thermistor probe as a guide, it is important to remember that the absence of a temperature rise may give a false sense of security if it has not been adequately positioned.
When ablation is performed with an open-irrigated-tip catheter, monitoring of microbubble formation is not feasible due to the artifact created by the irrigating saline. We have found that assessment of esophageal temperatures can assist in terms of lesion delivery in this situation, provided the thermistor is constantly moved to the area corresponding to the posterior LA segment being ablated. While monitoring esophageal temperature, it is important to use a system that provides fine detail of temperature changes in the esophagus. Apart from monitoring esophageal temperatures, other strategies can be implemented to minimize esophageal damage when using an open-irrigated ablation catheter. These include limiting maximum power to 35 W, limiting lesion delivery to 20 seconds, and avoiding excessive contact pressure.
Presentation
The clinical manifestations of atrial-esophageal fistula occur within 2 to 4 weeks, with a mean of 12 days (16). Fever, leukocytosis, dysphagia, and neurological symptoms in patients with a recent catheter ablation procedure for AF should raise suspicion of an atrial-esophageal fistula. Patients should be told to contact their electrophysiologist if suggestive symptoms develop in the weeks following ablation. A high index of suspicion is important in order to minimize the time to diagnosis and therapy. The method of presentation is variable; in the initial reports, patients presented with nonspecific signs and symptoms, such as dysphagia, odynophagia, intermittent cardiac or neurological ischemia (due to air emboli or vegetation), fever, sepsis, and melena (16). In a larger series of nine patients, sepsis was seen in all, neurological symptoms in eight, overt gastrointestinal hemorrhage in three, and two patients presented with chest pain associated with ST-segment elevation on ECG. The diagnosis was made before death in only four of the nine patients. A second report of five cases of AEF (four undergoing surgical and one percutaneous ablation) reported survival in three patients (21). Patients in this series presented with high fever (3, 60%) and/or chest and epigastric pain (2, 40%). Leukocytosis was uniformly present. Neurological complications occurred in three cases (60%) with a delay of 5 to 40 hours after first symptoms.
Diagnosis and Management
MR or CT imaging of the chest, and head if neurological manifestations are present, is the imaging modality of choice if AEF is considered. This may demonstrate air localized within the intravascular space or free air in the mediastinum. It should be noted that esophagoscopy with gaseous insufflation should not be performed as this may predispose to further gaseous embolism (15). In fact, any instrumentation of the esophagus, including transesophageal echocardiogram (TEE), should be avoided.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree