The aim of this study was to assess the effect of diabetes mellitus (DM) and glycosylated hemoglobin (HbA 1c ) on the progression of atherosclerosis in postmenopausal women. A retrospective analysis of the Women’s Angiographic and Vitamin and Estrogen (WAVE) trial, a multicenter randomized trial on progression of atherosclerosis in postmenopausal women, was performed. Baseline and follow-up angiography was performed in 320 women. Minimum luminal diameter and average luminal diameter at baseline and follow-up were measured in 1,735 coronary segments. Measurements and adverse events were grouped on the basis of history of DM and HbA 1c . DM was associated with more total cardiac events but with similar rates of death or myocardial infarction. There were greater reductions in minimum luminal diameter and average luminal diameter in segments from patients with known DM (p <0.001) and with a baseline HbA 1c ≥6.5% (p = 0.002 and p = 0.004, respectively). The greater reductions in minimum luminal diameter and average luminal diameter in the higher HbA 1c strata were only in patients with known DM. More new lesions, however, appeared with baseline HbA 1c ≥5.7%, irrespective of a history of DM. In conclusion, the relation between DM and the progression of coronary narrowing in postmenopausal women is complex. Clinically apparent DM, not elevated HbA 1c alone, appears to promote the progression of established coronary lesions even in HbA 1c ranges diagnostic of pre-DM and DM. This raises the possibility that coronary narrowing of existing stenosis in women with DM may be due to negative remodeling, a complex process that might be less dependent on hyperglycemia than new lesion formation.
Diabetes mellitus (DM) is a major risk factor for myocardial infarction (MI) and coronary artery disease. When coronary artery disease and DM coexist, mortality reaches approximately 45% over 7 years and 75% over 10 years. The prevalence of DM and its attendant risk for cardiovascular mortality and morbidity have increased in the past 20 years, making an understanding of this association critical. Compared to its effect in men, DM appears to have an even greater impact on cardiovascular disease risk in women. In a 13-year prospective study, the incidence of major cardiovascular disease events per 1,000 person-years in subjects without DM was roughly sixfold greater in men than in women (11.6 vs 1.8). In contrast, in patients with DM, the difference between men and women was lost (36.3 vs 31.6). The pathogenetic mechanisms responsible for the enhanced risk for coronary heart disease associated with DM remain obscure. In particular, the role of hyperglycemia, the sine qua non of the disorder, has been explored. To this point, however, the results of 2 large randomized trials testing the impact of strict glycemic control on clinical cardiovascular events have been disappointing. This study was undertaken to explore the effect of glycemic control (as indicated by glycosylated hemoglobin [HbA 1c ] levels) on the progression of coronary narrowing and new lesion formation in postmenopausal women with and without clinical histories of DM.
Methods
The National Heart, Lung, and Blood Institute sponsored the Women’s Angiographic and Vitamin and Estrogen (WAVE) multicenter, randomized trial, designed to test the effect of hormone replacement therapy and antioxidant vitamins on the progression of atherosclerosis measured by quantitative coronary angiography (QCA) in postmenopausal women ( ClinicalTrials.gov identifier NCT00000555 ). After 3 years of careful follow-up, the study failed to show an effect of either intervention.
Women were recruited from July 1997 to August 1999 at 7 clinical sites in the United States and Canada. To be included, women had to (1) be >55 years of age, (2) have undergone bilateral oophorectomy, or (3) have a follicle-stimulating hormone level ≥40 mlU/ml. They further had to have undergone clinically driven coronary angiography showing ≥1 coronary lesion of 15% to 75% in a major coronary artery or a primary branch within 4 months of study entry. Intervention on this lesion was not allowed. Patients were excluded if they had uncontrolled DM or hypertension, creatinine levels >2.0 mg/dl, New York Heart Association functional class IV heart failure, or a recent MI within 4 weeks of study. Supplementary Table 1 lists all exclusion criteria.
Of screened patients, 423 were enrolled. Scheduled exit coronary angiograms were obtained in 320 (76%). Intercurrent cardiovascular events and refusal of the follow-up study accounted for the others. Primary source documents describing all intercurrent events were used by an independent clinical events committee made up of 4 investigators to determine the nature of the events.
The available data set from this trial provides information on baseline and follow-up clinical status. Blood analysis was performed at baseline and at ≥1 follow-up visit. Follow-up visits were scheduled at 24, 30, 36, or 42 months. Adverse events during the study period, including cardiac events, revascularization, death, MI, and hospitalizations, were also recorded. The clinical and quantitative coronary angiographic data recorded in the original investigation formed the basis for this analysis.
QCA was performed by an independent laboratory (Stanford University, Edwin L. Alderman, MD, director). Details of the conduct of QCA and the quality assurance methods used are provided elsewhere. Measurements were carried out on up to 10 proximal epicardial artery segments. Study-driven exit coronary angiography was performed in 320 patients a mean of 2.86 years (median 3.02) from the initial angiographic study. Care was taken to obtain as nearly identical views of the segments evaluated at entry as possible. At the baseline angiographic study, segments containing no lesion of ≤15% were considered normal. If on the exit angiogram, such a segment contained a coronary narrowing of >15%, it was considered a new lesion.
For analysis, coronary segments were grouped on the basis of the patients’ baseline HbA 1c . Minimum luminal diameter (MLD) and average luminal diameter (ALD) for each segment studied were treated as continuous variables and regarded as outcome variables. Change in MLD was defined as (MLD at follow-up) − (MLD at baseline). Change in ALD was defined as (ALD at follow-up) − (ALD at baseline). These changes were compared across the strata of HbA 1c using analysis of variance. A p value for linear trend was used.
The number and percentage of new lesions (narrowing of the coronary lumen by ≥15%) appearing in segments that were normal at baseline were regarded as a second outcome variable. Differences were tested for significance using contingency tables or Fisher’s exact test. The Cochran-Armitage trend test was used to analyze the relation between HbA 1c stratum and the development of new lesions. Whether a history of DM influenced the relation between HbA 1c and the progression of coronary narrowing or the development of new lesions were evaluated using Pearson’s correlation coefficient.
Results
Potentially important baseline differences between participants who reported DM at baseline and those who did not existed. Women with known DM were slightly younger (p = 0.054) and had a higher mean weight (p <0.001). They had on average greater hip (p <0.001) and waist (p <0.001) circumferences. Moreover, women with DM more often gave histories of heart failure (p = 0.007), previous MI (p <0.05), and exercise-induced chest discomfort (p = 0.07). They more frequently were receiving angiotensin-converting enzyme inhibitors (p <0.001) and miscellaneous antihypertensive agents (p = 0.006). Total cholesterol and low-density lipoprotein levels were similar between women with DM and those without. Those with DM had lower levels of high-density lipoprotein (p <0.001) and apolipoprotein A (p <0.001) and higher levels of serum triglycerides (p = 0.009). Levels of serum creatinine (p = 0.012) and C-reactive protein (p = 0.018) levels were significantly higher in women with DM.
Table 1 displays the same characteristics compared by strata of HbA 1c level. These strata were established in line with the criteria established by the American Diabetes Association. It is thus not surprising that 93.1% of women in the highest HbA 1c strata (≥6.5%) reported histories of DM, while only 6.8% of those in the lowest strata (<5.7%) were aware of such a diagnosis. In the intermediate strata (between 5.7% and 6.5%), designated by the American Diabetes Association as “pre-diabetes,” 28.0% reported an awareness of DM. Not surprisingly, the baseline characteristics of those with HbA 1c ≥6.5% differed from those in the lowest tier (<5.7%) in a nearly identical manner to the differences between patients with known DM and those without. Moreover, it can reasonably be suggested that women who denied having received diagnoses of DM at baseline but whose baseline HbA 1c was in the pre-DM (n = 23) or DM (n = 6) range by American Diabetes Association criteria could be assumed to have recent onset of their glucose intolerance.
| Variable | HbA 1c <5.7% | 5.7% ≤HbA 1c >6.5% | HbA 1c ≥6.5% | p Value | Overall |
|---|---|---|---|---|---|
| Patients (segments) | 148 (486) | 82 (356) | 88 (560) | 318 (1,402) | |
| Age (yrs) | 66.54 ± 8.60 | 65.51 ± 7.90 | 63.05 ± 8.19 | 0.008 | 65.31 ± 8.43 |
| Weight (kg) | 73.34 ± 14.02 | 79.17 ± 14.70 | 86.54 ± 17.07 | <0.001 | 78.50 ± 16.03 |
| Hip circumference (cm) | 108.54 ± 12.02 | 112.31 ± 11.19 | 116.01 ± 13.83 | <0.001 | 111.43 ± 12.64 |
| Waist circumference (cm) | 93.03 ± 12.50 | 95.65 ± 11.26 | 102.55 ± 12.71 | <0.001 | 96.13 ± 12.81 |
| Patients receiving hormone replacement therapy in trial | 48 (31%) | 40 (25%) | 69 (44%) | 0.02 | 157 (44%) |
| Social history | |||||
| Current drinker | 0.48 ± 0.50 | 0.45 ± 0.50 | 0.31 ± 0.47 | 0.095 | 0.43 ± 0.50 |
| Current smoker | 20.4% (20/98) | 35.7% (20/56) | 25.9% (14/54) | 0.114 | 26% (54/208) |
| Pack-years of smoking | 29.03 ± 25.87 | 29.90 ± 30.65 | 20.86 ± 20.73 | 0.136 | 27.15 ± 26.24 |
| Medical history | |||||
| DM | 6.8% (10/147) | 28% (23/82) | 93.1% (81/87) | <0.001 | 36.1% (114/316) |
| Hypertension | 72.8% (107/147) | 85.4% (70/82) | 81.6% (71/87) | 0.06 | 78.5% (248/316) |
| Stroke | 7.5% (11/147) | 13.4% (11/82) | 8% (7/87) | 0.3 | 9.2% (29/316) |
| Atrial fibrillation | 18.4% (27/147) | 19.5% (16/82) | 16.1% (14/87) | 0.838 | 18% (57/316) |
| Other cardiac arrhythmias | 26.5% (39/147) | 19.8% (16/81) | 21.8% (19/87) | 0.468 | 23.5% (74/315) |
| Heart failure | 16.3% (24/147) | 6.1% (5/82) | 27.6% (24/87) | <0.001 | 16.8% (53/316) |
| MI | 38.1% (56/147) | 40.2% (33/82) | 46% (40/87) | 0.491 | 40.8% (129/316) |
| Coronary intervention | 53.1% (78/147) | 51.2% (42/82) | 55.2% (48/87) | 0.875 | 53.2% (168/316) |
| Chest discomfort with exercise | 63.9% (53/83) | 63% (29/46) | 88.1% (37/42) | 0.011 | 69.6% (119/171) |
| Claudication | 14.3% (21/147) | 14.6% (12/82) | 17.2% (15/87) | 0.82 | 152% (48/316) |
| Medications | |||||
| Anticlotting agents | 87.1% (128/147) | 95.1 (78/82) | 89.8% (79/88) | 0.153 | 89.9% (285/317) |
| Lipid-lowering agents | 53.7% (79/147) | 61.0% (50/82) | 63.6% (56/88) | 0.282 | 58.4% (185/317) |
| Antiarrhythmic agents | 2.7% (4/147) | 1.2% (1/82) | 1.1% (1/88) | 0.665 | 1.9% (6/317) |
| Digoxin | 8.8% (913/147) | 3.7% (3/82) | 5.7% (5/88) | 0.292 | 6.6% (21/317) |
| β blockers | 62.6% (92/147) | 72.0% (59/82) | 58.0% (51/88) | 0.153 | 63.7% (202/317) |
| Angiotensin-converting enzyme inhibitors | 31.3% (46/147) | 45.1% (37/82) | 55.7% (49/88) | <0.001 | 41.6% (132/317) |
| Diuretics | 35.4% (52/147) | 36.6% (30/82) | 46.6% (41/88) | 0.207 | 38.8% (123/317) |
| Other blood pressure medications | 4.8% (7/147) | 11.0% (9/82) | 12.5% (11/88) | 0.079 | 8.5% (27/317) |
| Lipid levels | |||||
| Cholesterol (mg/dl) at baseline | 201.78 ± 42.12 | 198.16 ± 37.92 | 203.22 ± 48.62 | 0.731 | 201.24 ± 42.91 |
| Cholesterol (mg/dl) at 36 months | 184.29 ± 38.33 | 197.30 ± 43.96 | 188.0 ± 46.48 | 0.337 | 188.29 ± 41.85 |
| Triglycerides (mg/dl) at baseline | 148.22 ± 77.51 | 155.94 ± 76.24 | 173.36 ± 95.39 | 0.08 | 157.14 ± 82.91 |
| Triglycerides (mg/dl) at 36 months | 140.35 ± 77.90 | 163.78 ± 95.87 | 152.06 ± 71.02 | 0.381 | 148.69 ± 80.76 |
| HDL (mg/dl) at baseline | 53.01 ± 12.97 | 48.79 ± 10.52 | 49.19 ± 13.72 | 0.019 | 50.86 ± 12.72 |
| HDL (mg/dl) at 36 months | 53.85 ± 12.27 | 51.24 ± 13.51 | 48.66 ± 11.75 | 0.124 | 51.94 ± 12.5 |
| LDL (mg/dl) at baseline | 118.72 ± 39.32 | 119.35 ± 34.87 | 120.72 ± 41.68 | 0.933 | 119.42 ± 38.76 |
| LDL (mg/dl) at 36 months | 102.55 ± 35.97 | 110.18 ± 38.45 | 109.11 ± 42.27 | 0.554 | 106.01 ± 38.12 |
| Apolipoprotein A at baseline | 145.77 ± 23.16 | 138.01 ± 19.82 | 136.44 ± 24.46 | 0.004 | 141.14 ± 23.04 |
| Apolipoprotein B at baseline | 102.12 ± 26.20 | 103.91 ± 27.62 | 109.69 ± 28.87 | 0.124 | 104.68 ± 27.43 |
| Apolipoprotein C at baseline | 14.15 ± 5.77 | 14.18 ± 5.30 | 15.72 ± 6.76 | 0.118 | 14.59 ± 5.97 |
| Cystatin C at baseline | 1.19 ± 0.50 | 1.26 ± 0.65 | 125 ± 0.65 | 0.611 | 123 ± 0.59 |
| Creatinine at baseline | 0.80 ± 0.18 | 0.87 ± 0.20 | 0.90 ± 0.28 | 0.001 | 0.85 ± 0.22 |
| C-reactive protein at baseline | 0.54 ± 0.55 | 0.63 ± 0.62 | 0.67 ± 0.68 | 0.263 | 0.60 ± 0.61 |
| C-reactive protein at 36 months | 0.45 ± 0.49 | 0.55 ± 0.57 | 0.55 ± 0.51 | 0.544 | 0.50 ± 0.51 |
QCA demonstrated that women with known histories of DM (n = 154), compared to those without histories of DM (n = 267), had significantly greater decreases in MLD and ALD of coronary segments (ΔMLD −0.13 vs −0.02 mm, p <0.001; ΔALD −0.9 vs +0.01 mm, p <0.001). In addition, in women who reported DM at baseline, a higher percentage of initially nondiseased coronary segments developed new lesions during the study period (30.3% vs 17.8%, p <0.001).
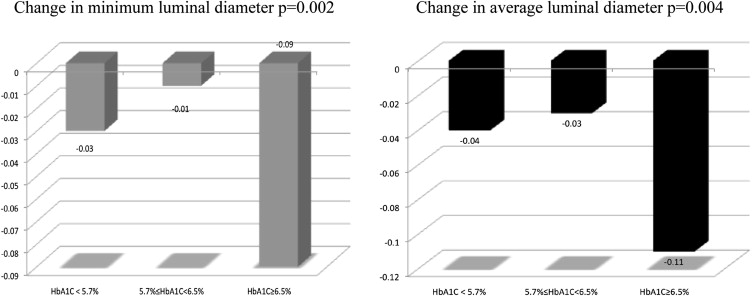
The progression of coronary narrowing was then examined on the basis of baseline HbA 1c strata. These results are listed in Table 2 and shown in Figure 1 . Baseline MLD and ALD of all examined segments were similar across the 3 strata of HbA 1c , but the degree of narrowing between baseline and exit angiograms for MLD and ALD was significantly greater in segments of patients with HbA 1c ≥6.5%. Moreover, lesions were found in segments that were normal at baseline much more frequently in the higher HbA 1c strata (p <0.001; Figure 2 ). The frequency of new lesions increased incrementally from one HbA 1c stratum to another.
| Variable | HbA 1c <5.7% | 5.7% ≤HbA 1c >6.5% | HbA 1c ≥6.5% | p Value |
|---|---|---|---|---|
| MLD at baseline (mm) | 2.2 | 2.15 | 2.15 | 0.447 |
| MLD at follow-up (mm) | 2.16 | 2.12 | 2.04 | 0.02 |
| Change in MLD (mm) | −0.04 | −0.03 | −0.11 | 0.002 ∗ |
| ALD at baseline (mm) | 2.59 | 2.58 | 2.58 | 0.891 |
| ALD at follow-up (mm) | 2.57 | 2.57 | 2.49 | 0.092 |
| Change in ALD (mm) | −0.03 | −0.01 | −0.09 | 0.004 ∗ |
∗ A p value for linear trend was calculated for change in MLD and ALD in each HbA 1c subgroup.
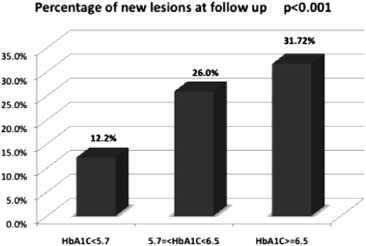
Because nearly all patients with baseline HbA 1c ≥6.5% had clinically diagnosed DM, we assessed the possibility that the significant changes in coronary narrowing observed in the higher HbA 1c strata might simply reflect the larger number of patients with established DM in those strata. Table 3 and Figures 3 and 4 demonstrate that the significant decreases in MLD and ALD observed in the higher HbA 1c strata were driven by histories of DM and were not apparent in patients without this diagnosis. Thus, the association between HbA 1c levels and the rate of coronary narrowing appeared to be absent in participants in whom the existence of abnormal glucose metabolism was not known before WAVE study entry, presumably DM of recent onset. Importantly, even in the absence of a clinically recognized glucose abnormality there was an incremental increase in the frequency of new lesions in the higher HbA 1c strata ( Figure 5 ). It seems possible that hyperglycemia has a stronger role in the initiation of new stenosis than in the progression of established coronary narrowing.
| Variable | HbA 1c <5.7% | p Value | 5.7% ≤HbA 1c >6.5% | p Value | HbA 1c ≥6.5% | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| History of diabetes (number of lesions) | No (542) | Yes (30) | No (291) | Yes (65) | No (91) | Yes (465) | |||
| MLD at baseline (mm) | 2.2 | 2.21 | 0.92 | 2.16 | 2.09 | 0.55 | 2.12 | 2.16 | 0.65 |
| MLD at follow-up (mm) | 2.17 | 2.1 | 0.62 | 2.16 | 1.92 | 0.03 ∗ | 2.12 | 2.02 | 0.22 |
| Change in MLD (mm) | −0.04 | −0.11 | 0.26 | 0 | −0.18 | 0.002 ∗ | 0.01 | −0.13 | 0.009 ∗ |
| ALD at baseline (mm) | 2.59 | 2.65 | 0.65 | 2.58 | 2.54 | 0.68 | 2.51 | 2.59 | 0.31 |
| ALD at follow-up (mm) | 2.57 | 2.55 | 0.88 | 2.61 | 2.39 | 0.03 ∗ | 2.49 | 2.48 | 0.45 |
| Change in ALD (mm) | −0.02 | −0.09 | 0.23 | 0.03 | −0.15 | <0.001 ∗ | 0.03 | −0.1 | 0.004 ∗ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


