6 Complete Atrioventricular Septal Defect
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria and left aortic arch.
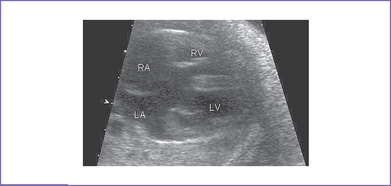
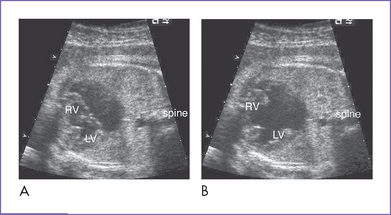
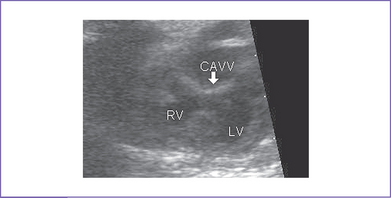
2. Complete atrioventricular septal defect (AVSD) (atrioventricular [AV] canal defect) with balanced ventricles (Figs. 6-1 to 6-3).
3. Both the aortic and pulmonary valves are of normal size for gestation and have normal Doppler velocity through them.
4. Mild right AV valve regurgitation (holosystolic) is present, with normal dP/dt (change in pressure per change in time) (800 mm Hg/s).
5. A cleft in the left AV valve is suspected.
6. The ductal and aortic arches are normal in size, with antegrade flow in both.
7. Tei index (myocardial performance index).
a. Right heart Tei index is 0.38.
b. Left heart Tei index is 0.37.
D. Fetal management and counseling
1. Management: Because of the possibility of aneuploidy, it is essential to obtain the fetal karyotype before completing counseling. The following information should be shared with the parents:
a. AVSD is associated with trisomy 21 in 60% to 70% of cases prenatally and postnatally.
b. A 22q11 microdeletion should be checked in AVSD because cases have been reported, although much less commonly than with trisomy 21.
c. In a series of fetuses with AVSD (Allan et al, 1994), there was a significant rate of intrauterine loss. This may be due, at least in part, to inclusion of fetuses with heterotaxy syndrome.
2. Follow-up: Serial antenatal visits at 4- to 6-week intervals.
a. AV valve regurgitation might increase in severity and can place the fetus at risk for development of congestive heart failure.
b. The mother will meet the surgeons, and details of the anatomy may be important to document at these visits. Valve morphology (dysplasia of valvar tissue) is important because the valve tissue at this age should be very thin. Inadequate AV valve tissue is associated with progressive AV valve regurgitation.
c. Systemic and pulmonary venous anomalies should be noted.
d. Change in the ventricular size due to redistribution of flow could mean the onset of an additional lesion, such as coarctation of the aorta.
e. Outflow tract obstruction can develop later in pregnancy as well, and may be progressive.
f. The left-sided AV valve cleft should be visualized in the short-axis view equivalent to confirm the diagnosis. The distance between the left ventricular (LV) papillary muscles should be assessed. A single LV papillary muscle can affect the results of a biventricular repair.
g. Single-ventricle palliation might be needed with unbalanced AVSD.
E. Delivery
1. If the fetus remains well compensated and the lesion is isolated, delivery can be vaginal at term in a secondary-level obstetrics center.
2. If the baby has trisomy 21 or there is any size discrepancy between the ventricles and the great arteries in the third trimester, one should be certain of the neonatal and cardiology capabilities of the delivering center.
F. Neonatal management
a. After delivery, the diagnosis is confirmed by echocardiography, and a cardiology consultation should be obtained.
b. Because coarctation of the aorta may be difficult to diagnose in utero with AVSD, a high index of suspicion is needed in the initial evaluation for this problem. The echocardiogram might have to be repeated after the ducts close in 2 to 3 days.
c. If the echocardiogram is normal and the blood pressures are normal in the upper and lower extremities at this time, then the neonate can be discharged and followed in the cardiology office.
d. These infants usually develop heart failure slowly and progressively over the first 4 to 8 weeks of postnatal life. Medical management (digoxin, diuretics, and captopril) is recommended for newborns with heart failure because surgical mortality is relatively high in this age group.
e. Pulse oximetry can vary from 85% to 95%. Oxygen is indicated only for saturations less than 85%. Oxygen is a potent pulmonary vasodilator and can worsen heart failure and cause respiratory arrest.
a. Palliative: Pulmonary artery (PA) banding may be carried out (with a relatively high risk) in small infants with no significant mitral regurgitation.
G. Follow-up
1. Postnatally, the prognosis for satisfactory repair of an AVSD is relatively good in children with Down syndrome and in those with normal chromosomes.
2. A small group of patients, often those with normal chromosomes, have recurrent left-sided lesions requiring repeated surgery or mitral valve replacement
3. The patient should receive subacute bacterial endocarditis (SBE) prophylaxis for up to 6 months after surgery. SBE prophylaxis should continue beyond 6 months if there is significant left or right AV valve regurgitation, residual VSD, or outflow tract obstruction
4. Patients with residual hemodynamic abnormalities might require anticongestive medications and some restriction of activities.
5. If the AVSD is severely unbalanced in pregnancy (usually with right ventricle [RV] dominance and LV hypoplasia), a one-ventricle or ventricle-and-a-half repair is offered. The long-term follow-up depends on the type of repair.
H. Risk of recurrence
1. When an AVSD is detected in association with a chromosome abnormality or a single gene disorder, the recurrence risk is determined by the underlying etiology. In most cases, concordance for the cardiac abnormality in case of recurrence is low.
2. Recurrence risk for trisomy 21.
a. The recurrence risk following the birth of a baby with Down syndrome depends on the fetal chromosome abnormality and the mother’s age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree