Subclinical atherosclerosis measured by coronary artery calcium (CAC) is associated with increased risk for multiple cardiovascular disease (CVD) outcomes and non-CVD death simultaneously. The aim of this study was to determine the competing risks of specific CVD events and non-CVD death associated with varying burdens of subclinical atherosclerosis. A total of 3,095 men and 3,486 women from the Multi-Ethnic Study of Atherosclerosis (MESA), aged 45 to 84 years, from 4 ethnic groups were included. Participants were stratified by CAC score (0, 1 to 99, and ≥100). Competing Cox models were used to determine competing cumulative incidences and hazard ratios within a group (e.g., those with CAC scores ≥100) and hazard ratios for specific events between groups (e.g., CAC score ≥100 vs 0). Risks were compared for specific CVD events and also against non-CVD death. In women, during a mean follow-up period of 7.1 years, the hazard ratios for any CVD event compared with a non-CVD death occurring first for CAC score 0 and CAC score ≥100 were 1.40 (95% confidence interval 0.97 to 2.04) and 3.07 (95% confidence interval 2.02 to 4.67), respectively. Coronary heart disease was the most common first CVD event type at all levels of CAC, and coronary heart disease rates were 9.5% versus 1.6% (hazard ratio 6.24, 95% confidence interval 3.99 to 9.75) for women with CAC scores ≥100 compared with CAC scores of 0. Similar results were observed in men. In conclusion, at all levels of CAC, coronary heart disease was the most common first CVD event, and this analysis represents a novel approach to understanding the temporal sequence of cardiovascular events associated with atherosclerosis.
The objective of our study was to determine the risks for diverse cardiovascular outcomes associated with subclinical coronary atherosclerosis. Standard survival models do not account for joint and competing risks for diverse outcomes; rather, specialized competing Cox models are required. Knowledge of first events is important, because emphasis on certain prevention strategies may be more effective for specific cardiovascular disease (CVD) outcomes. For example, clinical trials have documented significant reductions in the incidence of stroke and heart failure (HF) with antihypertensive therapy. Although global risk reduction is clearly indicated in patients with hypertension, other preventive measures, such as lipid lowering, smoking cessation, and antiplatelet therapy, have not reduced the risk for stroke to the same degree as antihypertensive therapy. Furthermore, the occurrence of 1 CVD event may markedly increase the risk for a subsequent event (e.g., myocardial infarction increases the risk for subsequent HF). An expert consensus document from the American Heart Association and American College of Cardiology Foundation stated that “it may be reasonable to consider use of CAC [coronary artery calcium] measurement in patients based on available evidence that demonstrates incremental risk prediction information in this selected (intermediate risk) patient group”. Since the time of that publication, CAC has been shown to improve risk prediction for coronary heart disease (CHD) even in lower risk populations. Thus, we sought to determine the first events in patients with no CAC and to compare competing risks for global CVD associated with subclinical atherosclerosis for men and women in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
MESA is a prospective cohort study supported by the National Heart, Lung, and Blood Institute, and details of its design have been published elsewhere. Participants aged 45 to 84 years were recruited from 6 field centers: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota. Participants were white (38%), African-American (28%), Hispanic (22%), and Chinese (12%). All participants gave written informed consent, and the study protocol was approved by the institutional review board at each site. Participants were free of clinical CVD at the time of the initial examination from July 2000 to August 2002.
Blood pressure was measured using a standard sphygmomanometer (Omron, Kyoto, Japan). Body mass index was defined as weight in kilograms divided by the square of height in meters. Blood samples were obtained after a 12-hour fast and analyzed for lipid measures and glucose as previously described. Medication use was determined by self-report. Diabetes mellitus was defined as fasting blood glucose >126 mg/dl or use of oral hypoglycemic agents. Current smoking was defined as cigarette smoking within the past 30 days.
The details of MESA’s computed tomographic scanning and interpretation protocols have been previously reported. CAC was assessed using chest computed tomography at the time of the baseline examination, concurrent with the collection of demographic, anthropometric, and clinical data. Either a cardiac-gated electron-beam computed tomographic scanner (Chicago, Los Angeles, and New York) or a multidetector computed tomographic scanner (Baltimore, Forsyth County, and St. Paul) was used. A cardiologist or radiologist interpreted all scans at a central reading center (Harbor-UCLA Medical Center, Los Angeles, California), and the Agatston score was calculated. We then stratified subjects into the following groups by CAC score, similar to what has been previously described : 0, 1 to 99, and ≥100.
The median follow-up time was 7.1 years. At 9- to 12-month intervals, interviewers contacted participants or family members by telephone to inquire about health status, hospitalizations, outpatient diagnoses of CVD, and deaths. All diagnoses were reviewed by 2 physician members of the MESA mortality and morbidity review committee. A CVD event was defined as the first occurrence of death due to CHD or nonfatal myocardial infarction (NFMI) (definite or probable), fatal and nonfatal stroke, transient ischemic attack, HF (definite or probable), or other CVD death (this consists of arrhythmic death not due to CHD and stroke, or peripheral vascular disease). The diagnosis of myocardial infarction was based on a combination of symptoms, electrocardiographic findings, and levels of cardiac biomarkers. The diagnosis of HF was predefined in MESA and was considered in the presence of clinical signs and symptoms, physician diagnosis and treatment, and imaging. Stroke was defined as a focal neurologic deficit lasting ≥24 hours or as a clinically relevant lesion on brain imaging if the duration of symptoms was <24 hours. Deaths were considered related to definite CHD if they occurred <28 days after myocardial infarctions, if participants had experienced chest pain within the 72 hours before death, or if the participants had histories of CHD and there were no known nonatherosclerotic, noncardiac causes of death. We included cases that were considered definite or probable but not “possible” cases. Deaths were considered not related to cardiovascular causes if other causes were suspected and confirmed by the review committee.
Baseline characteristics were compared by categorical CAC levels separately for men and women, using linear models for continuous variables and chi-square tests for categorical variables. All participants were followed until the occurrence of a CVD event, non-CVD death, or censoring for end of follow-up for a mean of 7.1 years. The CVD events considered in the analysis, including myocardial infarction and stroke, carry a high burden of morbidity and mortality; thus, we used non-CVD death as the comparison group because other non-CVD outcomes are unequal in terms of disability and years of life lost.
We calculated adjusted risks for CVD events separately for men and women stratified by degree of subclinical atherosclerosis. We then determined the first event occurring during the follow-up time, whether it was a CVD event or non-CVD death. In competing risks analysis, the occurrence of 1 type of event in a participant precludes consideration of any other event in that participant. If a CVD event occurred on the same day as the day of death, the CVD event was coded as occurring first. When multiple CVD events were diagnosed as occurring on the same date, we arbitrarily assigned one as occurring first. A patient was assigned with myocardial infarction as occurring before HF if both were diagnosed on the same date, and stroke was assigned as the first event if stroke and HF occurred on the same day. We used the data augmentation method as described by Lunn and McNeil to fit Cox proportional-hazards models for all CVD events combined compared with non-CVD death as a first event, separately in men and women and by degree of CAC. Standard Kaplan-Meier survival analyses are typically used in situations in which 1 event is considered, and the time to the event is considered the failure time. In the competing risks model we used in this study, a participant may fail from only 1 of the competing risks, and the time to the first event was considered the failure time. The hazards and the event-free survival are obtained from the augmented model, and the competing cumulative incidence rate is the product of these 2 quantities. Therefore, we were able to estimate hazard ratios and cumulative incidences for competing CVD events compared with non-CVD death within a given group (e.g., those with higher compared with lower burden of atherosclerosis).
In separate analyses, we used the method described by Fine and Gray to estimate the subdistribution hazard separately for men and women and by extent of atherosclerosis for each of 5 competing outcomes: (1) CHD death or NFMI, (2) HF, (3) fatal or nonfatal stroke or transient ischemic attack, (4) other cardiovascular death, and (5) noncardiovascular death. Fine and Gray’s model is a modified Cox proportional-hazards model that accounts for competing risks for different outcomes. The subdistribution hazards are modeled by keeping the competing risk observations in the risk set with diminishing weights. Thus, the effect estimated from Fine and Gray’s model shows the temporal differences between the 2 groups in terms of subdistribution hazards, reflecting the differing balance of event occurrences over time across the groups. This model also uses time-dependent covariates to model the nonproportionality of hazards. We stratified by gender and further adjusted for the effect of age and race. R version 2.10.1 and its competing risk library (R Foundation for Statistical Computing, Vienna, Austria) were used for these analyses. We used SAS version 9.2 (SAS Institute Inc., Cary, North Carolina) to compute event rates for any CVD event using standard Cox models, so that we could compare hazard ratios obtained from standard and competing Cox models. We also calculated competing risk models for the outcomes of cardiovascular and noncardiovascular death. Using 2-sided inference testing, p values <0.05 were considered statistically significant.
Results
The MESA sample for this analysis included 3,095 men and 3,486 women aged 45 to 84 years, and 1,870 men and 1,368 women had detectable CAC. Baseline characteristics of the study participants are listed by gender and stratum of CAC score in Table 1 . Adverse levels of traditional CVD risk factors were associated with the presence and extent of CAC.
| Variable | Men, Stratified by CAC Score (n = 3,095) | Women, Stratified by CAC Score (n = 3,486) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n = 1,217) | 1–99 (n = 905) | 100 (n = 973) | p Value | 0 (n = 2,109) | 1–99 (n = 838) | 100 (n = 539) | p Value | |
| Age (yrs) | 57.0 ± 9.0 | 62.6 ± 9.6 | 67.8 ± 8.9 | <0.001 | 58.4 ± 9.1 | 65.4 ± 9.6 | 71.0 ± 7.9 | <0.001 |
| Race | <0.001 | <0.001 | ||||||
| Caucasian | 365 (30%) | 348 (39%) | 504 (51%) | 738 (35%) | 323 (39%) | 256 (47%) | ||
| Chinese | 158 (13%) | 121 (14%) | 102 (10%) | 236 (11%) | 112 (14%) | 58 (11%) | ||
| African-American | 379 (31%) | 229 (26%) | 186 (19%) | 628 (30%) | 217 (26%) | 146 (27%) | ||
| Hispanic | 303 (25%) | 193 (22%) | 187 (19%) | 478 (23%) | 174 (21%) | 82 (15%) | ||
| Systolic blood pressure (mm Hg) | 121.3 ± 17.4 | 126.6 ± 19.1 | 130.9 ± 20.2 | <0.001 | 122.7 ± 22.0 | 130.6 ± 23.1 | 137.8 ± 24.0 | <0.001 |
| Body mass index (kg/m 2 ) | 27.5 ± 4.3 | 27.8 ± 4.4 | 28.1 ± 4.6 | 0.02 | 28.6 ± 6.2 | 28.7 ± 6.1 | 28.6 ± 6.0 | 0.95 |
| Total cholesterol (mg/dl) | 186.9 ± 33.7 | 189.7 ± 35.6 | 188.2 ± 35.8 | 0.2 | 197.9 ± 35.1 | 201.9 ± 35.8 | 204.4 ± 36.3 | 0.001 |
| High-density lipoprotein cholesterol (mg/dl) | 45.0 ± 11.1 | 44.8 ± 11.8 | 45.5 ± 12.7 | 0.47 | 56.9 ± 15.3 | 55.1 ± 15.2 | 56.0 ± 15.5 | 0.01 |
| Fasting blood glucose (mg/dl) | 96.3 ± 27.4 | 100.3 ± 34.7 | 104.1 ± 36.3 | <0.001 | 92.2 ± 25.4 | 97.2 ± 29.5 | 99.4 ± 29.2 | <0.001 |
| Physical activity (METs-min/week) | 6,957 ± 6,797 | 6,507 ± 7,576 | 5,917 ± 5,941 | 0.002 | 5,501 ± 5,105 | 4,747 ± 4,988 | 4,254 ± 3,920 | <0.001 |
| Education less than high school | 195 (16%) | 142 (16%) | 148 (15%) | 0.18 | 366 (18%) | 185 (22%) | 119 (22%) | <0.001 |
| Annual income <$35,000 | 407 (35%) | 308 (36%) | 367 (39%) | 0.13 | 907 (45%) | 454 (57%) | 327 (65%) | <0.001 |
| Diabetes mellitus | 114 (9%) | 117 (13%) | 183 (19%) | <0.001 | 172 (8%) | 105 (13%) | 96 (18%) | <0.001 |
| Current smokers | 190 (16%) | 131 (15%) | 124 (13%) | <0.001 | 240 (12%) | 97 (12%) | 67 (12%) | <0.001 |
| Antihypertensive medications | 254 (21%) | 262 (29%) | 412 (42%) | <0.001 | 589 (28%) | 345 (42%) | 266 (49%) | <0.001 |
| Lipid-lowering therapy | 119 (10%) | 144 (16%) | 220 (22%) | <0.001 | 224 (11%) | 198 (24%) | 139 (26%) | <0.001 |
| History of cancer | 57 (5%) | 76 (9%) | 97 (10%) | <0.001 | 141 (7%) | 74 (9%) | 70 (13%) | <0.001 |
| Liver disease | 55 (5%) | 33 (4%) | 41 (4%) | 0.62 | 65 (3%) | 21 (3%) | 9 (2%) | 0.16 |
| Lung disease | 109 (9%) | 81 (9%) | 85 (9%) | 0.94 | 270 (13%) | 98 (12%) | 67 (12%) | 0.70 |
| Renal disease | 18 (1%) | 23 (3%) | 25 (3%) | 0.13 | 46 (2%) | 19 (2%) | 11 (2%) | 0.95 |
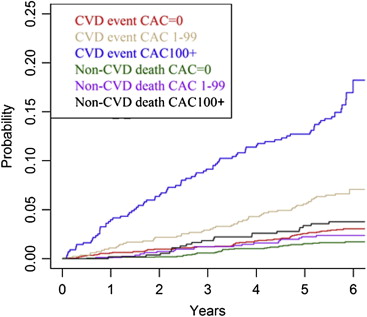
During a mean follow-up period of 7.1 years (46,159 person-years), there were 320 CVD events and 157 non-CVD deaths in men and 212 CVD events and 112 non-CVD deaths in women. Figures 1 and 2 show the incidence of first CVD events and non-CVD death in men and women by stratum of CAC score. Table 2 lists competing cardiovascular outcomes and noncardiovascular deaths in men and women by strata of CAC score, using the method of Lunn and McNeil. In men, within each stratum of CAC score, even a CAC score of 0, any CVD event was the more likely first event, compared with a non-CVD death. The cumulative incidence of any CVD event occurring first in men with no detectable CAC was 4.0%, and the incidence of non-CVD death was 3.1%, for a hazard ratio of a CVD event occurring first of 1.35 (95% confidence interval [CI] 0.87 to 2.11). The relative magnitude of the hazard ratio for any CVD event compared with non-CVD death was higher in participants with CAC scores of 1 to 99 and ≥100.
| Variable | Hazard Ratio and Competing Cumulative Incidence in Men and Women | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| 0 (n = 1,217) | 1–99 (n = 905) | ≥100 (n = 973) | 0 (n = 2,109) | 1–99 (n = 838) | ≥100 (n = 539) | |
| Hazard ratio (95% CI) for CVD event vs non-CVD death within group | 1.35 (0.87–2.11) | 1.68 (1.18–2.38) | 2.60 (1.99–3.41) | 1.40 (0.97–2.04) | 1.58 (1.04–2.40) | 3.07 (2.02–4.67) |
| Competing incidence by stratum of CAC score | ||||||
| Non-CVD death | 3.1% | 6.6% | 8.5% | 2.4% | 4.7% | 7.3% |
| CHD death/NFMI | 1.8% | 6.0% | 13.9% | 1.6% | 4.1% | 9.5% |
| Fatal/nonfatal stroke | 1.2% | 2.7% | 3.2% | 1.2% | 3.0% | 5.5% |
| HF | 1.0% | 1.0% | 3.0% | 0.5% | 0.2% | 3.1% |
| Other CVD death | 0% | 0.1% | 0.3% | 0% | 0.2% | 0.4% |
| Any CVD event | 4.0% | 9.8% | 20.3% | 3.2% | 7.6% | 18.5% |
In women, any CVD event was most likely to occur first across all strata of CAC, rather than non-CVD death, as listed in Table 2 . Similar to men, the relative magnitude of the hazard ratio was higher with increasing burden of CAC. We conducted additional analyses adjusting the hazard ratios obtained from the method of Lunn and McNeil for CVD risk factors (results not shown). The overall pattern was similar, but the estimates were less stable.
As listed in Table 3 , we used the method of Fine and Gray to show that the hazard ratio of any CVD event occurring first in men with the highest levels of CAC compared with no CAC was 5.69 (95% CI 4.13 to 7.85). Analysis of specific CVD events in men showed that CHD or NFMI was the most likely first CVD event across all strata of CAC. The cumulative incidences of CHD or NFMI occurring first in men with the highest levels of CAC and in those with no CAC were 13.9% and 1.8%, respectively, for a hazard ratio of 8.31 (95% CI 5.25 to 13.2). Men with CAC scores ≥100 were significantly more likely to experience HF as a first event, compared with those with no CAC. In women with the highest levels of CAC compared with women with no CAC, the hazard ratio for CHD or NFMI was 6.24 (95% CI 3.99 to 9.75). Similar to men, women with the highest levels of CAC had significantly greater hazards for experiencing HF as a first event. Models 2 and 3 in Table 3 show that the hazard ratios for any CVD event were modestly attenuated by adjustment for age and race and then additionally for CVD risk factors.



