Restriction of compartment size
Increased compartment volume
From hemorrhage
Fractures
Casts
Vascular injury
Splints
Drugs (anticoagulants)
Burn eschar
Hemophilia; sickle cell
Tourniquets
From muscle edema/swelling
Tight dressings
Crush – trauma, drugs, or alcohol
Fracture reduction
Rhabdomyolysis/blast injury
Closure of fascial defects
Sepsis
Incomplete skin release
Exercise induced
Military antishock trousers
Envenomation or bee sting
Prolonged extrication trapped limb
Massive resuscitation
Localized external pressure
Intracompartmental fluid infusion
Long leg brace
Phlegmasia cerulea dolens
Automated BP monitoring
Electrical burns
Malpositioning on OR table
Reperfusion injury
Postpartum eclampsia
As pressures increase because of internal or external forces, venous flow decreases and narrows the arteriovenous perfusion gradient, resulting in diminished tissue blood flow. This condition is self-perpetuating, leading to a continuous loop that must be broken with the timely initiation of definitive care [7]. Hippocrates may have been the first to describe the dangers of elevated intracompartmental pressures in 400 BC [47]. When untreated, permanent deformity of the distal extremity results, a phenomenon first described by Richard von Volkmann in the late nineteenth century [48]. In 1926, Jepson [49] was able to experimentally create CS in the extremities of dogs using external pressure (Esmarch’s bandage), and he suggested drainage of the compartments would be of value in preventing deformity.
Cellular anoxia is the final common pathway of all compartment syndromes. However, the interrelation between increased compartment pressure, blood pressure, and loss of tissue perfusion leading to cell death are incompletely understood. This incomplete understanding leads to diagnostic and treatment challenges. As ischemia continues, irreparable damage to tissue ensues and myoneural necrosis occurs. Development of CS depends on many factors, including the duration of the pressure elevation, the metabolic rate of the tissues, vascular tone, associated soft tissue damage, and local blood pressure [6]. Nerves demonstrate functional abnormalities (paresthesias and hypoesthesia) within 30 min of ischemic onset. Irreversible functional loss will occur after 12–24 h of total ischemia [1]. The muscle shows functional changes after 2–4 h of ischemia with irreversible loss of function beginning at 4–12 h.
A dynamic relationship exists among the blood pressure, the level of intracompartmental pressure (ICP), and the duration of time for which a raised pressure is maintained. It is known that the higher the pressure, the faster and greater is the damage, but a lower pressure maintained for a longer period of time may also cause similar tissue damage [38]. Many authors [50, 51] have concluded that catastrophic clinical results were inevitable if fasciotomies were delayed for over 12 h, whereas a full recovery was achieved if decompression was performed within 6 h of making the diagnosis. Compartment syndromes lasting longer than 8–12 h are likely to produce chronic functional deficits, such as contractures, sensory aberrations, and motor weakness. Clinically, a precise pressure threshold and duration do not exist above which significant damage is irreversible and below which recovery is assured.
Tissue that has been previously subjected to intervals of ischemia is especially sensitive to increased pressure. Bernot and colleagues [52] showed that tissue previously compromised by ischemia prior to elevated ICP has a lower threshold for metabolic deterioration and irreversible damage. It must be kept in mind that polytrauma patients with low blood pressures can sustain irreversible injury at lower compartment pressures than patients with normal blood pressures, and a very high index of suspicion should be maintained in this group.
Epidemiology/Risk Factors
Given the consequences of missing a CS, it is important to identify the population at risk, as well as factors which predict the occurrence of this condition. In a 10-year retrospective review of over 10,000 trauma patients sustaining extremity injury, Branco et al. described a fasciotomy rate of 2.8 % [35]. During this period, 315 fasciotomies were performed on 237 patients with 68.4 % done below the knee, 14.4 % on the forearm, and 8.9 % on the thigh (see Fig. 4.1). In a review of 294 combat-injured soldiers undergoing 494 fasciotomies, Ritenour et al. reported the calf as the most common site (51 %) followed by the forearm (22.3 %), the thigh (8.3 %), the upper arm (7.3 %), the hand (5.7 %), and the foot (4.8 %) [53].
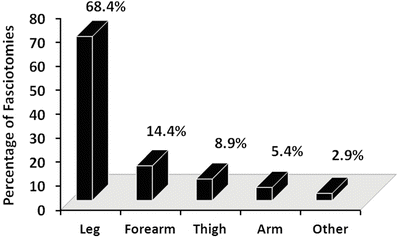

Fig. 4.1
Anatomic distribution of 315 fasciotomies done for extremity trauma. Other includes the foot and hand (Adapted, with permission from Elsevier, from Branco et al. [35])
Certain injury patterns have been associated with higher likelihood of needing fasciotomy. Blick et al. found a close association between grade of fracture, degree of comminution, and risk of development of CS in a retrospective review of 198 open tibia fractures [54]. Abouezzi et al. found a 28 % incidence of fasciotomy in patients with peripheral vascular injuries treated at a level I trauma center. They determined that injury to popliteal vessels was more likely (62 % cases) to result in fasciotomy than above the knee vascular injury (19 % cases) [55]. This finding was echoed by Gonzalez et al. [56] who reported that CS of the lower extremity was more likely to be associated with penetrating injuries below the knee (94 %) than above the knee. Another study evaluated femoral vascular injuries in particular and found that the rates of fasciotomy depended on whether there was isolated arterial (13 % fasciotomy) or venous injury (3 % fasciotomy) or a combination (38 % fasciotomy) [57].
Branco et al. [35] found that the incidence of fasciotomy varied widely by mechanism of injury (0.9 % after motor vehicle collision to 8.6 % after a gunshot wound). Additionally the need for fasciotomy was related to the type of injury ranging from 2.2 % incidence for patients with closed fractures up to 41.8 % in patients with combined venous and arterial injuries (see Fig. 4.2). The study by Branco identified the risk factors associated with the need for fasciotomy after extremity trauma: young males with penetrating or multisystem trauma, requiring blood transfusion, and with open fractures, elbow or knee dislocations, or vascular injury (arterial, venous, or combined) are at the highest risk of requiring a fasciotomy after extremity trauma [35]. Taylor et al. [39] reiterate that age is a major prognostic factor with patients less than 35 more likely than older patients to develop CS following the same type of injury. These orthopedic authors list tibial shaft fractures as the most common antecedent cause (36 %) followed by soft tissue injury (23 %), distal radial fracture (9.8 %), crush syndrome (7.9 %), diaphyseal fracture of the radius/ulna (7.9 %), femoral fracture (3.0 %), and tibial plateau fracture (3.0 %) [39].
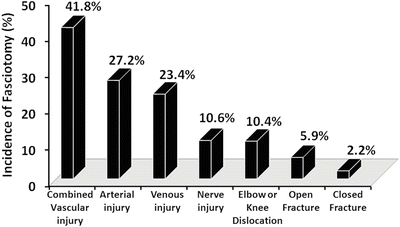

Fig. 4.2
Incidence of fasciotomy as a result of the injury type in 315 fasciotomies done for extremity trauma (Adapted, with permission from Elsevier, from Branco et al. [35])
Diagnosis
Diagnosis depends on a high clinical suspicion and an understanding of risk factors, pathophysiology, and subtle physical exam findings. The diagnosis of CS is often based on subtle changes in symptoms and vague clinical exam findings. The physician must have a high degree of suspicion when treating these patients. Time to diagnosis is the most important prognostic factor for these patients. Insufficient understanding of the natural history and limited evaluation of signs and symptoms primarily account for delays in diagnosis [39]. It is important to realize that the aim is to recognize and treat raised intracompartmental pressure before irreversible cell damage occurs [47].
Numerous authors have stated that the diagnosis of CS is a clinical diagnosis [4, 6, 7, 33, 34, 39, 47, 58–62]. The classically described five “Ps” – pain, pallor, paresthesias, paralysis, and pulselessness – are said to be pathognomonic of CS. However, these are usually late signs and extensive and irreversible injuries may have taken place by the time they are manifested. In the earliest stages of CS, patients may report some tingling and an uncomfortable feeling in their extremity followed closely by pain with passive stretching of the muscles of the affected compartment. The most important symptom of CS is pain greater than expected due to the injury alone.
Nerve tissue is affected first by the subsequent end-tissue hypoxia causing pain on passive motion seen early in the development of CS, sparing distal pulses until late in the course [33]. The loss of pulse is a late finding, and the presence of pulses and normal capillary refill do not rule out CS! The presence of open wounds does not exclude CS. In fact, the worst open fractures are actually more likely to have a CS.
All clinical signs have inherent drawbacks in making the diagnosis. Pain is an unreliable and variable predictor. It can range from being very mild to severe and is already present in patients who have suffered acute trauma [63]. The pain of the obvious injury can mask that of an impending CS and cannot alone be depended upon to make the diagnosis [60]. Mubarak and colleagues [64] found that pain in response to passive stretching of the affected muscle compartment was also an unreliable sign and suggested that the presence of hypoesthesia was more dependable. However, Rorabeck and Macnab [65] found hypoesthesia to be the last clinical finding to develop as the syndrome progressed.
Although all have a role in the diagnosis of CS, the constellation of signs and symptoms and overall clinical picture are more important than the presence or absence of any particular finding [39]. Ulmer [41] undertook a review of over 1,900 articles on CS published in the English literature between 1966 and 2001 to assess whether published studies support basing the diagnosis of CS of the lower leg on clinical findings. This exhaustive review produced only four studies in which sensitivity, specificity, and positive and negative predictive values could be calculated [66–69]. Data from these studies suggest that the sensitivity of clinical findings for diagnosing CS is low (13–19 %). The positive predictive value of clinical findings was 11–15 %, and the specificity and negative predictive value were each 97–98 %. These findings suggest that the clinical features of CS are more useful by their absence in excluding the diagnosis than when they are present in confirming the diagnosis. When one clinical finding was present, the probability of a CS was 25 and 93 % when three clinical findings were present [41].
Given the findings of Ulmer’s review and the fact that clinical findings may be absent in patients with altered sensorium, under the influence of drugs or alcohol, distracting injuries, or paralysis, many authors advise using tissue pressure measurements as an adjunct to clinical findings [36, 60, 64, 70]. Others advocate the use of compartment pressure measurement as a principle criterion for the diagnosis of CS [68, 71].
In actual practice, tissue pressure (compartment pressure) measurements have a limited role in making the diagnosis of CS. However, in polytrauma patients with associated head injury, drug and alcohol intoxication, intubation, spinal injuries, use of paralyzing drugs, extremes of age, unconsciousness, or low diastolic pressures, measuring compartment pressures may be of use in determining the need for fasciotomy.
The pressure threshold for making the diagnosis of CS is controversial. A number of authors recommend 30 mmHg [64], and others cite pressures as high as 45 mmHg. Ouellete [72] recommended that an ICP of 15–25 mmHg should be used in patients with clinical signs and greater than 25 mmHg for those without. Many surgeons use the “Delta-P” system. The compartment pressure is subtracted from the patient’s diastolic blood pressure to obtain the Delta-P. Whitesides as early as 1975 proposed that the muscle was at risk when the ICP was within 10–30 mmHg of the diastolic pressure [73]. If the Delta-P is less than 30, the surgeon should be concerned that a CS may be present. For instance, if the diastolic blood pressure was 60 mmHg and the measured compartment pressure was 42 mmHg, the “Delta-P” would be 18 (60 − 42 = 18) and the patient is likely to have CS. Other factors to consider when considering fasciotomy are the length of time of transport to definitive care and ability to do serial exams.
One of the earliest champions of measuring compartment pressures was Whitesides [74], who used an 18-gauge needle inserted into the compartment connected to a mercury manometer to obtain pressure measurements. This and other methods to include the wick catheter technique developed by Mubarek [2], and the slit catheter technique developed by Rorabeck [75] have been widely used in the past, but suffer from the cumbersome nature of setting them up and user variability. A commercially available portable handheld, self-contained, electronic pressure monitor with a digital display is available (Stryker® intracompartmental pressure monitor system, Stryker® Surgical, Kalamazoo, Michigan) has replaced most of these less reproducible devices as the current standard (Fig. 4.3). Another commercially available device, Twin Star ECS® that was initially developed to remove fluid from compartments by tissue ultrafiltration [76] has recently received clearance to be used a continuous pressure monitor as well (Fig. 4.4). An alternative approach is to use an 18-gauge needle attached to a side-port arterial line setup inserted into the compartment.
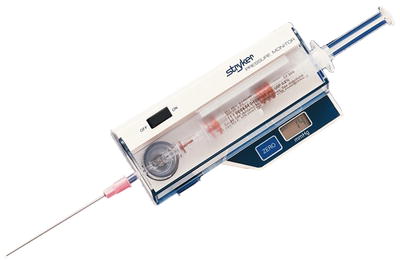
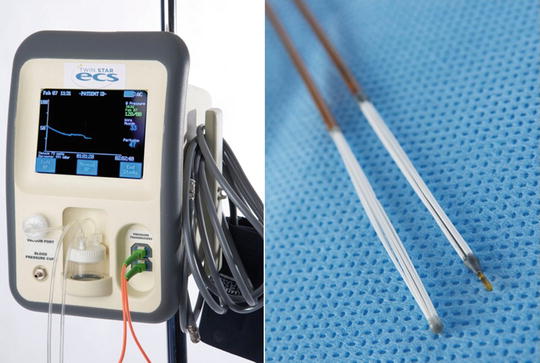

Fig. 4.3
Intracompartmental pressure monitor system manufactured by Stryker® Surgical, Kalamazoo, Michigan (Courtesy of Stryker® Instruments. Kalamazoo, Michigan)

Fig. 4.4
Intracompartmental pressure monitor system and catheter tip manufactured by Twin Star Medical (Twin Star ECS®), Minneapolis, MN
The use of pressure measurements to decide if fasciotomy is necessary can be very useful if significantly elevated, but there are several potential pitfalls when interpreting pressure measurements. It is important to keep in mind that the pressure in one compartment can be normal while that in the compartment immediately adjacent can be elevated. It is therefore essential to have thorough understanding of the anatomy of the compartments and the confidence that the pressure of all of the compartments of the suspicious extremity have been measured prior to making any conclusions about a normal pressure. Additionally, there may be variations of pressure within a compartment at different levels. Seiler et al. [77] demonstrated that there is significant intracompartmental variability within normal compartments, and Heckman et al. [70] showed that ICP measurements also show variability within an injured compartment.
There have been a variety of other noninvasive techniques proposed for making the diagnosis of CS to include near-infrared spectroscopy [78–80], laser Doppler flowmetry [81], pulsed phase-locked loop ultrasound [82–84], magnetic resonance imaging [85, 86], skin quantitative hardness measurement [87, 88], vibratory sensation [89, 90], and scintigraphy using 99Tcm-methoxyisobutyl isonitril (MIBI) [91]. Though some of these techniques have shown early promise, none have reached clinical use outside of protocols [92].
At the end of the day, CS remains primarily a clinical diagnosis fueled by a high index of suspicion and supported by objective examination findings. The reliance on clinical examination with a low threshold for fascial release may result in unwarranted fasciotomies, but it avoids the grave consequences of a missed diagnosis.
Treatment
The definitive treatment of CS is early and aggressive fasciotomy. In patients with vascular injury in whom a fasciotomy in conjunction with a vascular repair is planned, it makes great sense to perform the fasciotomy before doing the repair. The rationale for this is that the ischemic compartment is likely to already be tight and thus will create inflow resistance to your vascular repair, making it susceptible to early thrombosis.
It is imperative that surgeons caring for traumatically injured patients fully understand the anatomy of the extremity compartments and the technique of fasciotomy for each. It is extremely embarrassing for the surgeon and life altering for the patient if an adequate or timely fasciotomy is not done and the patient loses the limb as a result. As previously mentioned in the series reported by Feliciano et al., 75 % of amputations of the lower extremity were related to a delay in performing, or performing an incomplete fasciotomy [41]. In a recent large review of combat patients, Ritenour et al. reported that patients who had incomplete or delayed fasciotomy had twice the rate of major amputation and three times the rate of mortality [53]. In spite of these alarming numbers, many otherwise well-trained surgeons continue to make these mistakes. The following section will focus on the recommended technique for performing fasciotomy of the lower extremity emphasizing the landmarks, relevant anatomy, and pitfalls.
Fasciotomy of the Lower Leg
As previously discussed, the lower leg (calf) is the most common site for CS requiring fasciotomy. The preferred technique for fasciotomy of the below the knee CS is the two-incision four-compartment fasciotomy. This technique was widely used in WWII and was the standard treatment for decompression in a syndrome involving more than one compartment [93]. An alternative single-incision approach in which the fibula is resected has been championed by some [94], but has been condemned by others as being unnecessarily mutilating, more likely to result in injury to the peroneal nerve, and likely to result in incomplete release of the compartments [3, 4]. Because it uses a lateral approach, the one-incision technique protects the great saphenous vein from injury during the fasciotomy.
In 2014, the standard approach to treating CS of the lower extremity in traumatically injured patients is the two-incision, four-compartment fasciotomy as popularized by Mubarak and Owen in 1977 [3]. As previously discussed, this procedure is not performed frequently by the majority of general or even vascular surgeons [45, 46], and the rate of delayed, incomplete, or improperly performed fasciotomy is alarmingly high with preventable morbidity and mortality as a result [53]. The most commonly missed compartments are the anterior followed closely by the deep posterior [53], and this likely occurs as a result of incomplete knowledge of the anatomy of the lower extremity. Successful fasciotomy of the lower extremity requires a thorough understanding of the anatomy and the relevant landmarks. The lower leg has four major tissue compartments bounded by investing the muscle fascia (Fig. 4.5). It is important to understand the anatomical arrangement of these compartments as well as some key structures within each compartment in order to perform a proper four-compartment fasciotomy (Fig. 4.6).
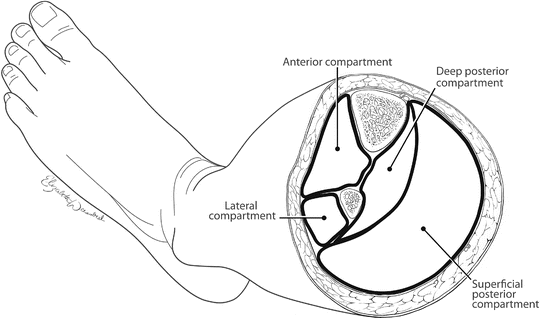
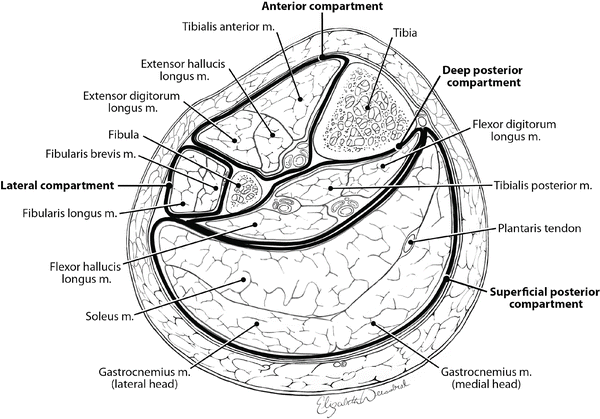

Fig. 4.5
Cross-sectional anatomy of the midportion of the left lower leg depicting the four compartments that must be released when performing a lower leg fasciotomy

Fig. 4.6
Cross-sectional anatomy of the midportion of the left lower leg depicting the contents of the four compartments and their relationship to the tibia and fibula
It is not necessary to remember the names of all the muscles (Fig. 4.6) in each compartment, but it is useful to remember the following: the anterior compartment contains the anterior tibial artery and vein and the deep peroneal nerve; the lateral compartment, the superficial peroneal nerve (which must not be injured); the superficial posterior compartment, the soleus and gastrocnemius muscles; and the deep posterior compartment, the posterior tibial and peroneal vessels and the tibial nerve (Fig. 4.7).
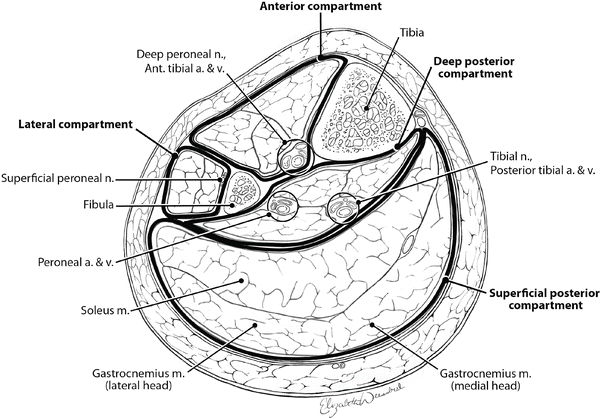

Fig. 4.7
Cross-sectional anatomy of the midportion of the left lower leg depicting the key structures and relationships that must be kept in mind when performing a two-incision four-compartment fasciotomy
There are several key features that will enable performance of a successful two-incision four-compartment fasciotomy. Proper placement of the incisions is essential. As extremities needing fasciotomy are often grossly swollen or deformed, marking the key landmarks will aid in placement of the incisions. The tibial spine serves as a reliable midpoint between the incisions. The lateral malleolus and fibular head are used to identify the course of the fibula on the lateral portion of the leg (Fig. 4.8). The lateral incision is usually made just anterior (~1 fingerbreadth) to the line of the fibula, or a finger in front of the fibula. It is important to stay anterior to the fibula as this minimizes the chance of damaging the superficial peroneal nerve. The medial incision is made one thumb breadth below the palpable medial edge of the tibia, or a thumb below the tibia (Fig. 4.9). The extent of the skin incision should be approximately two fingerbreadths below the tibial tuberosity and above the malleolus on either side.
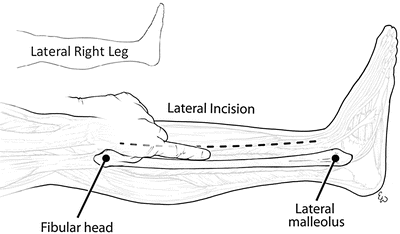
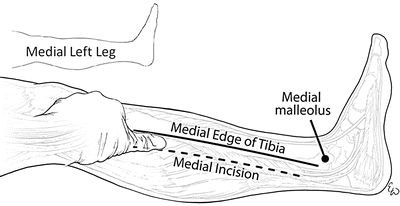

Fig. 4.8
The fibular head and lateral malleolus are as used reference points to mark the edge of the fibula, and the lateral incision (dotted line) is made one finger in front of the fibula

Fig. 4.9
The medial incision (dotted line) is made one thumb breadth below the palpable medial edge of the tibia (solid line)
It is very important to mark the incisions on both sides prior to opening them, as the landmarks of the swollen extremity will become distorted once the incision is made.
The Lateral Incision of the Lower Leg
The lateral incision (Fig. 4.8) is made one finger in front of the fibula and should in general extend from two fingerbreadths below the head of the fibula down to two fingerbreadths above the lateral malleolus. The exact length of the skin incision will depend on the clinical setting, and care must be taken to make sure that it is long enough such that the skin does not serve as a constricting band. The skin and subcutaneous tissue are incised to expose the fascia encasing the lateral and anterior compartments. Care should be taken to avoid the lesser saphenous vein and peroneal nerve when making these skin incisions.
Once the skin flap is raised, the intermuscular septum is sought and identified. This is the structure which divides the anterior and lateral compartments. In the swollen or injured extremity, it may be difficult to find the intermuscular septum. In these circumstances, the septum can often be found by following the perforating vessels down to it (Fig. 4.10). Classically the fascia of the lower leg is opened using an “H”-shaped incision (Fig. 4.11). This will be accomplished by making the crosspiece of the “H” using a scalpel which will expose both compartments and the septum. The legs of the “H” are made with curved scissors using just the tips which are turned away from the septum to avoid injury to the peroneal nerve (Figs. 4.11 and 4.12). It is important to identify the intermuscular septum and open the fascia at least one centimeter from it on either side, because the terminal branch of the deep peroneal nerve perforates the septum in the distal one third of the lower leg and this could be cut if care is not taken. The anterior and lateral compartments are then fasciotomized 1 cm in front and behind the intermuscular septum.
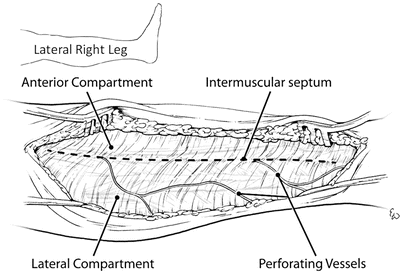
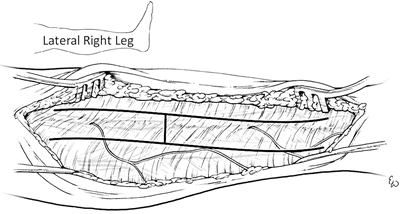
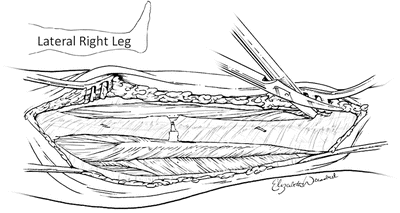

Fig. 4.10
The intermuscular septum separates the anterior and lateral compartments and is where the perforating vessels traverse. This is a representation of the lateral incision of the right lower leg

Fig. 4.11
The fascia overlying the anterior and lateral compartments is opened in an “H”-shaped fashion

Fig. 4.12
The fascia overlying the anterior and lateral compartments is opened in an “H”-shaped fashion using scissors with the tips turned away from the septum
The fascia should be opened by pushing the partially opened scissor tips in both directions on either side of the septum opening the fascia from the head of the fibula down to the lateral malleolus in a line that is 1–2 cm from the septum. Inspection of the septum and identification of the deep peroneal nerve and/or the anterior tibial vessels confirm entry into the anterior compartment. The skin incision should be closely inspected and extended as needed to ensure that the ends do not serve as a point of constriction.
As previously stated, the anterior compartment is the one most commonly missed during lower-extremity fasciotomy. One of the reasons for missing the anterior compartment stems from making the incision too far posteriorly, either directly over or behind the fibula. When the incision is made in this manner, the septum between the lateral and the superficial compartment may be directly below the incision and is erroneously identified as the septum between the anterior and lateral compartments (Fig. 4.13). When the lateral incision is made one finger in front of the fibula, the intramuscular septum between the anterior and lateral compartments is found directly below the incision making successful decompression likely (Fig. 4.14).