Angiographic evaluation of intermediate left main coronary artery stenosis (LMS) is often limited. Three-dimensional (3D) quantitative coronary angiography has recently developed to overcome 2-dimensional (2D) quantitative coronary angiographic (QCA) limitations. In patients with angiographically intermediate LMS, we investigated whether 3D quantitative coronary angiography was superior to 2D quantitative coronary angiography in predicting the presence of a significant LMS, defined as a minimum luminal area <6 mm 2 at intravascular ultrasound (IVUS). 2D and 3D quantitative coronary angiography were compared in their measurements of minimum luminal area, percent area stenosis, minimum luminal diameter, and percent diameter stenosis and in their prediction of an IVUS minimum luminal area <6 mm 2 . In total 58 target lesions were interrogated, 25 (43%) of which had an IVUS minimum luminal area <6 mm 2 . Correlation between 3D-QCA minimum luminal area and IVUS minimum luminal area was stronger than the correlation between 2D-QCA minimum luminal area (or minimum luminal diameter) and IVUS minimum luminal area (R = 0.67, p = 0.0001, and R = 0.40, p = 0.001, respectively, p = 0.04 for comparison). To predict IVUS minimum luminal area <6 mm 2 , the most accurate 2D-QCA measurement was minimum luminal diameter (area under curve 0.81, cutoff 2.2 mm, p = 0.0001), and the most accurate 3D-QCA measurement was minimum luminal area (area under curve 0.86, cutoff 5.6 mm 2 , p = 0.0001). 2D-QCA percent diameter stenosis did not significantly predict IVUS minimum luminal area <6 mm 2 (area under curve 0.56, cutoff 38%, p = 0.45). In conclusion, the accuracy of quantitative coronary angiography in predicting LM IVUS minimum luminal area <6 mm 2 is limited. When IVUS is not available or contraindicated, 3D quantitative coronary angiography may assist in the evaluation of intermediate LMS. Among 2D-QCA parameters, minimum luminal diameter is more accurate than percent diameter stenosis in predicting significant LMS.
A correct evaluation of the severity of atherosclerotic left main coronary artery stenosis (LMS) remains paramount to establish an appropriate management strategy and significantly affects patient prognosis. Visual assessment of LMS by standard coronary angiography is often suboptimal because of anatomic constraints such as vessel overlap, tortuosity, and foreshortening. Accordingly, in the entire coronary tree, the LM is the segment with less interoperator agreement in the assessment of stenosis significance. Two-dimensional (2D) quantitative coronary angiography, introduced in the late 1980s as a more objective and reproducible approach, has in turn revealed its limitations. Recently, 3-dimensional (3D) quantitative coronary angiographic (QCA) reconstructions, based on the fusion of ≥2 2D angiographic views, have been introduced in the effort to overcome some well-known limitations of 2D quantitative coronary angiography. Intravascular ultrasound (IVUS) provides high-resolution cross-sectional images of the coronary lumen, with direct visualization of the vessel wall and precise estimation of plaque area, and the usefulness of IVUS in the assessment of angiographically ambiguous LM lesions has been largely demonstrated. The aim of this study was to compare 2D- and 3D-QCA evaluations of intermediate LMS using IVUS assessment as the anatomic gold standard.
Methods
We retrospectively identified 80 consecutive patients in the IVUS database of a tertiary referral hospital (Agostino Gemelli Hospital, Catholic University of the Sacred Heart, Rome, Italy) with de novo angiographically intermediate LMS (40% to 80% diameter stenosis by angiography) who underwent IVUS evaluation from January 2009 through November 2010. Exclusion criteria were (1) clinical reasons such as ongoing ST-segment elevation myocardial infarction, previous coronary artery bypass graft surgery, and cardiogenic shock; and (2) angiographic reasons such as extremely tortuous coronary arteries, lack of ≥2 angiographic projections separated by 30°, diffusely diseased LM with no obvious angiographic reference segment. A figure detailing the study flow of patients is presented in the Supplementary Material ( Supplementary Figure 1 ). From the total of 80 screened patients, 12 were excluded because of an inability to perform 2D and 3D quantitative coronary angiography (9 and 8, respectively). Major reasons for 2D-QCA inability were diffuse LM disease and extreme tortuosity, and 3D quantitative coronary angiography could not be performed because of lack of suitable views and diffuse LM disease. Fifty-eight patients with intermediate LMS were deemed suitable for analysis.
In detail, 1 experienced interventional cardiologist (F.B.) analyzed all available angiographic projections targeting the LM (because of the intermediate nature of the stenosis, an average of 5 ± 2 views were acquired) and selected 2 electrocardiographically gated end-diastolic frames in 2 unmagnified views (1 in each view) separated from each other by ≥30° (in the lateral or craniocaudal plane). Care was taken to select 2 frames with minimal foreshortening and vessel overlap. Two views were selected, 1 with the most severe angiographic stenosis and another with the least foreshortening (visual estimation for the 2 views). The filled catheter had to be present on the image. One selected view was marked as the view with the most “severe” angiographic stenosis at visual estimation. The “spider” view (left caudal angulation) was excluded because of the well-known high degree of foreshortening. These 2 views were then used by different analysts for 2D quantitative coronary angiography (only the most severe view) and 3D quantitative coronary angiography (the 2 views) in blinded fashion.
All patients gave their informed consent, and the study was approved by the ethics committee of the Catholic University of the Sacred Heart.
Angiographic cine images were acquired at 15 frames/s. LMSs were angiographically classified into the following 3 groups: (1) ostial lesion, within 3 mm of the aortic orifice; (2) distal lesion, within 3 mm of the bifurcation; and (3) mid lesion, >3 mm from the ostium and distal bifurcation. 2D quantitative coronary angiography was performed by a skilled analyzer (M.C.) blinded to the results of 3D-QCA and IVUS analysis using standard commercial software (CASS QCA 5.9, Pie Medical Imaging BV, Maastricht, the Netherlands) on a single selected 2D end-diastolic image frame (described earlier) according to a standard protocol. Vessel diameters were calculated in absolute values (millimeters), after manual calibration with the outer diameter of the contrast-filled nontapered part of the guiding catheter, and comparison of the computed mean catheter diameter in pixels with the known catheter size in millimeters. For contour detection, the user indicated the vessel by choosing 2 center positions proximal and distal to the stenosis. Vessel contours were automatically determined. Edge detection correction was performed if required. Reference vessel diameter was based on the computer estimation of the original arterial dimensions at the stenosis site ( Figure 1 ). The reference segment diameter for mid-LM lesions was measured from angiographically normal segments proximal and distal to the stenosis; in patients with an ostial stenosis, a distal angiographically normal segment was analyzed; for distal LMS comprising the ostia of the left anterior descending coronary artery and/or left circumflex coronary artery, we used the single-vessel approach, meaning that the analysis was extended toward the vessel explored by IVUS. However, a proximal angiographically normal segment was considered a reference for these patients. The following angiographic parameters were obtained: minimal lumen diameter (millimeters), reference vessel diameter (millimeters), and percent diameter stenosis. We defined significant stenosis as a diameter stenosis >50%. Further parameters also were obtained: reference vessel area (square millimeters) and minimum luminal area (square millimeters) derived using the circle formula from diameters and percent area stenosis.
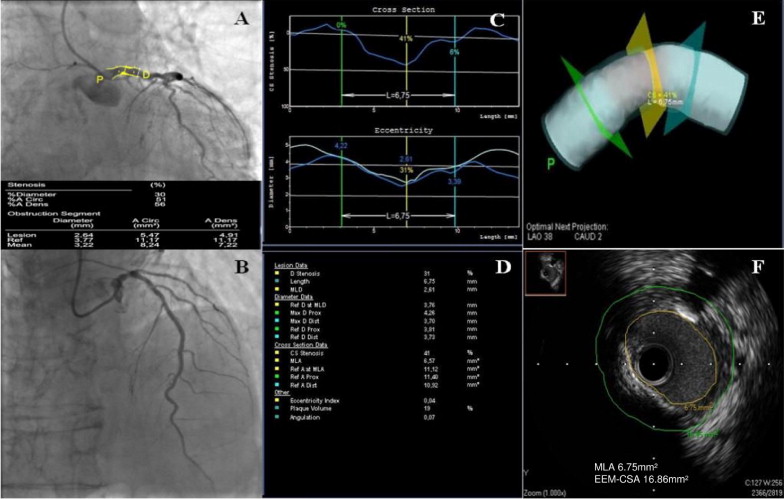
3D quantitative coronary angiography was performed offline by 2 skilled angiographers (I.D. and D.T.) who were unaware of 2D-QCA and IVUS analyses using a dedicated 3D reconstruction software (CardioOp-B system, Paieon Medical, Ltd., Rosh Ha’Ayin, Israel). The 2 best selected orthogonal angiographic views of the target lesion in the electrocardiographically gated end-diastolic frame were used for 3D-QCA reconstruction. After calibration performed in the same frame as 2D quantitative coronary angiography, the site of the minimum luminal diameter and proximal and distal coronary artery segments were manually identified; on the second view, after manual positioning of the marker at the site of minimum luminal diameter, an epipolar map was automatically generated, and the operator manually placed proximal and distal markers within the boundaries of the map. The software then automatically generated a 3D representation of the arterial lumen ( Figure 1 ). In patients with an ostial stenosis, a distal angiographically normal plane was considered a reference, whereas in patients with distal stenosis, we used a proximal segment. Minimum luminal diameter, reference vessel diameter, percent diameter stenosis, minimum luminal area, reference vessel area, and percent area stenosis were measured.
IVUS studies were performed with a 40-MHz frequency mechanical probe (Atlantis Pro SR, Boston Scientific, Natick, Massachusetts). After intracoronary administration of nitroglycerin 100 μg, the IVUS catheter was advanced into left anterior descending coronary artery (46 patients) or left circumflex coronary artery (12 patients) according to the angiographic evaluation of plaque extension, and an imaging run was performed back to the aorta using an automated transducer pullback at 0.5 mm/s. When a lesion was in an ostial location, the guiding catheter was disengaged from the coronary artery during IVUS imaging. A skilled interpreter (I.P.) blinded to results of 2D and 3D quantitative coronary angiography performed IVUS analysis according to the American College of Cardiology Clinical Expert Consensus Document on Standards Acquisition, Measurement and Reporting of IVUS. The following parameters were measured: minimum luminal area, obtained by tracing the intimal leading edge; external elastic membrane cross-sectional area, obtained by tracing the external elastic membrane cross-sectional area at the lesion site; plaque plus media cross-sectional area, obtained by the difference between the external elastic membrane cross-sectional area and the minimum luminal area ( Figure 1 ). The reference segment was a normal-looking LM cross section located proximal or distal to the stenosis; in patients with a mid-LM lesion, the average of measurements proximal and distal to the stenosis was selected. We used the value of 6 mm 2 as the threshold minimum luminal area for significant LMS.
2D and 3D quantitative coronary angiograms were compared in their measurements of minimum luminal diameter, percent diameter stenosis, minimum luminal area, and percent area stenosis and in their prediction of significant IVUS minimum luminal area (<6 mm 2 ) of LMS. Continuous variables were expressed as mean ± SD and compared with analysis of variance. Categorical variables were expressed as frequency and compared with chi-square test. Normality of data was determined using the D’Agostino–Pearsons test and verified using histographic plots. Correlations were calculated using the Pearson correlation coefficient for parametric data. Comparison between the Pearson R coefficients was performed with Fisher’s r-to-z transformation using MedCalc 11.4.4 (MedCalc Software, Mariakerke, Belgium). Bland–Altman plots were used to analyze the agreement among 2D and 3D quantitative coronary angiography and IVUS. Paired t tests were used to assess significant differences between 2D- and 3D-QCA measurements. Receiver operating curve analysis was used to determine the sensitivity and specificity of 2D and 3D quantitative coronary angiography to predict significant IVUS LMS. Paired comparisons between receiver operating curves were performed within patient groups using MedCalc. Other statistical analyses were performed using SPSS 17 (SPSS, Inc., Chicago, Illinois). A 2-sided p value of 0.05 was considered statistically significant.
Results
In total 58 LMSs were interrogated in 58 patients: 6 (10%) were significant by 2D quantitative coronary angiography (defined as diameter stenosis ≥50% ), whereas 33 (57%) and 25 (43%) were significant by 3D-QCA and IVUS analyses, respectively (defined as minimum luminal area <6 mm 2 ). LMSs were classified according to lesion site in the ostium, mid-shaft, and distally and were 17 (29%), 5 (9%), and 36 (62%), respectively. Patients’ characteristics were similar among the 3 LM lesion groups ( Table 1 ). Similarly, there were no significant differences among LMSs classified according to lesion site in any 2D-QCA, 3D-QCA, and IVUS measured variable ( Table 2 ).
| Variables | All LMSs (n = 58) | Site of LMS | p Value | ||
|---|---|---|---|---|---|
| Ostium (n = 17) | Mid (n = 5) | Distal (N = 36) | |||
| Age (years) | 67.8 ± 9 | 67.2 ± 8.3 | 72.6 ± 7.5 | 67.4 ± 9.6 | 0.47 |
| Men | 47 (81%) | 13 | 4 | 30 | 0.84 |
| Previous myocardial infarction | 12 (21%) | 4 | 1 | 7 | 0.94 |
| Systemic hypertension | 48 (83%) | 16 | 5 | 27 | 0.13 |
| Hypercholesterolemia | 29 (50%) | 9 | 4 | 16 | 0.32 |
| Current smoker | 9 (16%) | 3 | 1 | 5 | 0.57 |
| Ex-smoker | 20 (35%) | 4 | 2 | 14 | |
| Diabetes mellitus | 14 (24%) | 5 | 0 | 9 | 0.4 |
| Family history | 13 (22%) | 3 | 1 | 9 | 0.83 |
| Acute coronary syndromes | 32 (55%) | 8 | 4 | 20 | 0.43 |
| Number of coronary artery with ≥1 significant stenosis | 0.07 | ||||
| 0 | 8 (14%) | 4 | 0 | 4 | |
| 1 | 16 (27%) | 5 | 1 | 10 | |
| 2 | 22 (38%) | 7 | 1 | 14 | |
| 3 | 12 (21%) | 1 | 3 | 8 | |
| Variables | All LMSs (n = 58) | Site of LMS | p Value | ||
|---|---|---|---|---|---|
| Ostium (n = 17) | Mid (n = 5) | Distal (n = 36) | |||
| 2-Dimensional quantitative coronary angiographic parameters | |||||
| Minimum luminal diameter (mm) | 2.39 ± 0.52 | 2.52 ± 0.47 | 2.42 ± 0.45 | 2.33 ± 0.54 | 0.49 |
| Diameter stenosis (%) | 34 ± 10 | 30 ± 13 | 33 ± 9 | 35 ± 9 | 0.20 |
| Minimum luminal area (mm 2 ) | 4.70 ± 2 | 5.13 ± 1.85 | 4.72 ± 1.72 | 4.50 ± 2.11 | 0.57 |
| Area stenosis (%) | 55 ± 14 | 50 ± 18 | 55 ± 11 | 57 ± 11 | 0.16 |
| 3-Dimensional quantitative coronary angiographic parameters | |||||
| Minimum luminal diameter (mm) | 2.37 ± 0.62 | 2.44 ± 0.8 | 2.51 ± 0.6 | 2.32 ± 0.52 | 0.72 |
| Diameter stenosis (%) | 35 ± 12 | 37 ± 15 | 30 ± 10 | 34 ± 10 | 0.38 |
| Minimum luminal area (mm 2 ) | 5.94 ± 2.86 | 6.65 ± 3.45 | 6.37 ± 4.15 | 5.55 ± 2.34 | 0.41 |
| Area stenosis (%) | 45 ± 15 | 48 ± 13 | 44 ± 18 | 44 ± 15 | 0.61 |
| Intravascular ultrasound parameters | |||||
| Minimum luminal area (mm 2 ) | 7.29 ± 2.92 | 8.06 ± 3.77 | 6.08 ± 1.94 | 7.10 ± 2.53 | 0.34 |
| External elastic membrane cross-sectional area (mm 2 ) | 18.63 ± 5.77 | 18.50 ± 6 | 15.66 ± 1.82 | 19.1 ± 6 | 0.46 |
Overall, there were significant but moderate correlations between 2D and 3D quantitative coronary angiography for minimum luminal diameter (R = 0.48, p = 0.0001), reference vessel diameter (R = 0.67, p = 0.0001), minimum luminal area (R = 0.62, p = 0.0001), reference vessel area (R = 0.70, p = 0.0001), and percent area stenosis (R = 0.31, p = 0.02), whereas there was a nonsignificant correlation for percent diameter stenosis (R = 0.22, p = 0.09). Paired comparison of 2D versus 3D quantitative coronary angiography yielded smaller minimum luminal area (4.70 ± 2 vs 5.94 ± 2.86 mm 2 , p = 0.0001), larger percent area stenosis (55 ± 14. vs 45 ± 15%, p = 0.0001), and similar minimum luminal diameter (2.39 ± 0.52 vs 2.37 ± 0.62 mm, p = 0.79) and percent diameter stenosis (34 ± 10 vs 35 ± 12%, p = 0.63) for 2D quantitative coronary angiography. In the subgroup analysis according to lesion site, differences were mainly confined to the distal group (minimum luminal area 4.50 ± 2.11 vs 5.55 ± 2.34, p = 0.004; percent area stenosis 57 ± 11 vs 44 ± 15, p = 0.0001; Supplementary Table 1 ). Bland–Altman plots demonstrating limits of agreement among 2D-QCA, 3D-QCA, and IVUS minimum luminal area are shown in Figure 2 .
When lesions were dichotomized according to IVUS minimum luminal area <6 or >6 mm 2 , there were significant differences in all measured angiographic parameters, except for percent diameter stenosis on 2D and 3D quantitative coronary angiograms and percent area stenosis on 2D quantitative coronary angiogram ( Table 3 ). Of all 3D-QCA measurements, only minimum luminal diameter and minimum luminal area correlated with IVUS minimum luminal area (R = 0.54, p = 0.0001; R = 0.67, p = 0.0001). Of all 2D-QCA measurements, only minimum luminal diameter and minimum luminal area correlated with IVUS minimum luminal area (R = 0.40, p = 0.0001). The correlation between 3D-QCA minimum luminal area and IVUS minimum luminal area was significantly stronger than the correlation between 2D-QCA minimum luminal area (or minimum luminal diameter) and IVUS minimum luminal area (p = 0.04), whereas there was no significant difference between 3D-QCA and 2D-QCA minimum luminal diameter correlations ( Table 4 ).



