QRS duration (QRSd) is used to diagnose left bundle branch block (LBBB) and is important to determine cardiac resynchronization therapy eligibility. The same QRSd thresholds established decades ago are used for all patients. However, significant interpatient variability of normal QRSd exists, and individualized QRSd thresholds might improve diagnosis and intervention strategies. Previous work reported left ventricular (LV) mass and papillary muscle location predicted QRSd in healthy subjects, but the relation in diseased ventricles is unknown. The aim of the present study was to determine the association between LV anatomy and QRSd in patients with cardiomyopathy. Patients referred for primary prevention implantable defibrillators (n = 166) received cardiac magnetic resonance imaging, and those with normal conduction (without bundle branch or fascicular block) and LBBB were studied. The LV mass, length, internal diameter, LV end-diastolic volume, septal and lateral wall thicknesses, and papillary muscle location were measured. In patients with normal conduction, LV length (r = 0.35, p <0.001), mass (r = 0.32, p <0.001), diameter (r = 0.20, p = 0.03), and septal wall thickness (r = 0.20, p = 0.03) had positive correlations with QRSd. In patients with LBBB, LV length (r = 0.32, p = 0.03), mass (r = 0.39, p = 0.01), diameter (r = 0.34, p = 0.02), and LV end-diastolic volume (r = 0.32, p = 0.04) had positive correlations with QRSd. Contrary to previous studies in healthy subjects, papillary muscle angle (location) was not associated with QRSd in cardiomyopathy patients with normal conduction or LBBB. In conclusion, increasing LV anatomical measurements were associated with increasing QRSd in patients with cardiomyopathy. Future work should investigate the use of LV anatomical measurements in developing individualized QRSd thresholds for diagnosing conduction abnormalities such as LBBB and identifying candidates for cardiac resynchronization therapy.
Conventional QRS duration (QRSd) thresholds for diagnosis of cardiac disease were established decades ago, and the same thresholds are still used for all patient types. For example, QRSd thresholds were used to diagnose left bundle branch block (LBBB) as well as to determine patient eligibility for treatments such as cardiac resynchronization therapy (CRT). Because QRSd interpatient variability could make diagnosis difficult, determining an individualized baseline QRSd could aid in determining the onset and progression of cardiac conditions. Causes of QRSd variability include differences in cardiac anatomy. Previous work in healthy subjects demonstrated that left ventricular (LV) mass, length, and papillary muscle location predicted QRSd. Previous studies also showed that QRSd prolongation is associated with LV structural changes including increases in wall thickness. Other anatomical factors that influence QRSd include fiber orientation, LV geometry, the complexity of the Purkinje network, and the shape of the LV wall. However, it is not known if LV anatomical characteristics correlate with QRSd in patients with cardiomyopathy referred for primary prevention implantable defibrillators or CRT. The aim of the present study was to evaluate the association between LV anatomy and QRSd in patients with cardiomyopathy, specifically those with normal conduction and those with LBBB.
Methods
The Johns Hopkins Hospital Institutional Review Board and the Food and Drug Administration Research in Human Subjects Committee approved the study. Patients were referred to the Johns Hopkins Medical Institutions for implantable cardioverter-defibrillator (ICD) placement for primary prevention of sudden cardiac death and were enrolled from November 2003 to December 2010. This was a retrospective analysis of the cardiovascular magnetic resonance (CMR) arm of the Prospective Observational Study of Implantable Cardioverter Defibrillators study, which has been previously described. The inclusion and exclusion criteria were described previously. In brief, patient inclusion required (1) left ventricular ejection fraction ≤35% measured by a clinically indicated non-CMR study (echocardiography, nuclear scintigraphy, or ventriculography), (2) coronary angiography, (3) no other indications for ICD placement (e.g., syncope, sustained ventricular arrhythmias, or cardiac arrest), and (4) no contraindications to CMR (e.g., existing cardiac device). Patients were classified as having ischemic cardiomyopathy if they had a history of myocardial infarction or revascularization, evidence of coronary artery stenosis >50% of the left main or proximal left anterior descending coronary arteries, or >50% stenosis of 2 or more epicardial vessels. Other patients were classified as having nonischemic cardiomyopathy. Patients with acute myocarditis, congenital heart disease, hypertrophic cardiomyopathy, and infiltrative heart disease were excluded. Patients were divided into normal His-Purkinje conduction (normal conduction) and LBBB using the criteria discussed later. Patients not matching the defined normal conduction or LBBB classes and those with right bundle branch block or left anterior fascicular block were excluded from the analysis.
Clinically indicated 12-lead electrocardiograms (ECGs) were acquired with a GE-Marquette system before ICD implantation as previously described. ECGs were analyzed by 2 observers and classified in consensus based on the following criteria : (1) normal conduction—QRSd <120 ms and (2) LBBB—QRSd ≥140 ms (men) or 130 ms (women), QS or rS in V1 with mid-QRS notching/slowing in ≥2 of the leads I, aVL, V1, V2, V5, or V6. The new strict LBBB criteria, with different thresholds for men and women, were proposed previously because men have larger ventricles and longer QRSd than women. Multiple independent studies have shown that they improve prediction of which patients’ benefit from CRT, likely identifying which patients have a true LBBB.
Of the 235 primary prevention patients with ICD available for analysis, 167 met the inclusion criteria for the present study and had analyzable data. One patient with LBBB was excluded because his LV mass was >8 SDs and QRSd was >5 SDs from the mean of other patients with LBBB (subject LV mass = 525.30 g, QRSd = 244 ms).
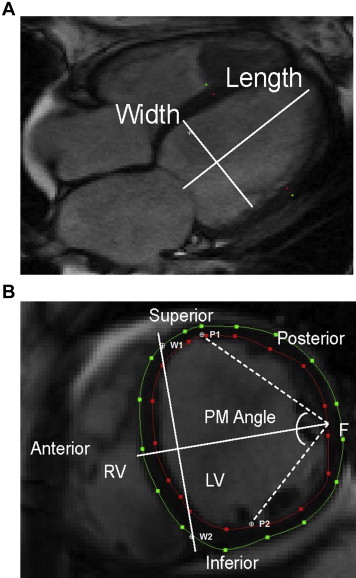
The cine and CMR protocol were described previously. In summary, patients underwent imaging with a 1.5-T scanner (Signa CV/I; GE Healthcare Technologies, Wisconsinor Avanto; Siemens Medical Systems, Erlangen, Germany). LV mass and left ventricular end-diastolic volume (LVEDV) measurements were performed with CINEtool (GE Healthcare, Wisconsin), as previously described. All images were analyzed subsequently using the image analysis program Segment (Segment 1.9; Medviso AB, Lund, Sweden) to determine the LV papillary muscle angle, LV length, LV diameter, ventricular septal wall thickness, and LV lateral wall thickness ( Figure 1 ). Papillary muscle angle was measured, as previously described, in the short-axis view in the plane where the papillary muscle inserts into the LV wall ( Figure 1 ). LV diameter and wall thicknesses were measured at this level at end-diastole. LV length was measured from the 4-chamber long-axis view ( Figure 1 ). In brief, the papillary muscle angle was determined by defining anterior and posterior papillary muscle insertion points (P1 and P2) along with the ventricular septal wall border points (W1 and W2), where the right ventricle meets the left ventricle. A perpendicular bisector line was then drawn between the W1 and W2 segments and was used to determine the free wall midpoint (F). Two segments (P1F and P2F) were drawn between the midpoint (F) and the papillary muscle insertion points (P1 and P2). The papillary muscle angle was defined as the angle between P1F and P2F segments.
Baseline patient characteristics were compared among the ECG conduction types with one-way analysis of variance (Kruskal-Wallis). Associations between LV anatomical measurements and QRSd were assessed by linear regression. Categorical variables were evaluated by the chi-square test. All statistical analyses were performed using SigmaPlot (version 11.0; Systat Software, Inc., California). p Values <0.05 were considered statistically significant.
Results
The 166 patients had a mean age of 56 years, were 23% women, had a mean left ventricular ejection fraction of 28%, had 49% ischemic cardiomyopathy etiology, and had a distribution of New York Heart Association heart failure classes (23% class I, 48% class II, and 29% class III). Patients with LBBB, n = 44, compared with patients with nonbundle/fascicular block, n = 122, were older; were predominantly women; had smaller LV scar size, higher QRSd, less ischemic; and had a higher New York Heart Association heart failure class ( Table 1 ). Patients with LBBB also had larger LV mass, LV length, and LVEDV but smaller LV wall thicknesses. No significant difference was seen in the LV diameter or papillary muscle angle between groups ( Table 1 ).
| Characteristics | Normal Conduction (n = 122) | LBBB (n = 44) | p Value |
|---|---|---|---|
| Age (yrs) | 54.4 ± 12.1 | 59.6 ± 11.5 | 0.01 |
| Women | 22 (18%) | 16 (36%) | 0.01 |
| LVEF (%) | 28.4 ± 9.0 | 25.4 ± 8.6 | 0.06 |
| Scar size (%LV) | 13.9 ± 13.4 | 6.6 ± 9.5 | 0.001 |
| QRSd (ms) | 98.2 ± 10.8 | 160.4 ± 16.2 | <0.001 |
| Ischemic etiology | 68 (56%) | 13 (30%) | 0.003 |
| New York Heart Association | <0.001 | ||
| Class I | 37 (30%) | 2 (5%) | |
| Class II | 64 (52%) | 15 (34%) | |
| Class III | 21 (17%) | 27 (61%) | |
| Body surface area (m 2 ) | 2.07 ± 0.3 | 2.04 ± 0.3 | 0.47 |
| LV length (mm) | 95.7 ± 9.8 | 101.0 ± 11.9 | 0.004 |
| LV diameter (mm) | 65.4 ± 8.8 | 67.7 ± 10.3 | 0.18 |
| LV mass (g) | 144.5 ± 45.2 | 159.3 ± 42.3 | 0.04 |
| Ventricular septal wall thickness (mm) | 7.1 ± 2.6 | 5.5 ± 2.3 | <0.001 |
| LV lateral wall thickness (mm) | 5.8 ± 2.2 | 4.7 ± 1.4 | 0.002 |
| Papillary muscle angle (°) | 108.8 ± 16.0 | 107.9 ± 14.0 | 0.73 |
| LVEDV (ml) | 236.7 ± 75.6 | 271.0 ± 95.2 | 0.04 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


