Bivalirudin, a direct thrombin inhibitor, is as effective as unfractionated heparin (UFH), with decreased bleeding in patients with acute coronary syndromes who undergo percutaneous coronary intervention (PCI). The aim of this study was to evaluate the effectiveness of bivalirudin versus UFH in selected PCI patients at high bleeding risk. Four hundred one consecutive patients who underwent PCI fulfilling ≥1 enrollment criterion (age >75 years, chronic renal failure, and diabetes mellitus) were randomized to bivalirudin (bolus 0.75 mg/kg followed by infusion during the procedure; n = 198) or UFH (75 IU/kg; n = 203). In the overall population, 39% were aged >75 years, 22% had renal failure, 63% had diabetes, and 29% had acute coronary syndromes. The primary efficacy end point was the 30-day incidence of major adverse cardiac events (cardiac death, myocardial infarction, stent thrombosis, or target vessel revascularization). The primary safety end point was the occurrence of any bleeding or entry-site complications after PCI. All patients were preloaded with clopidogrel 600 mg. Glycoprotein IIb/IIIa inhibitors were used at the operators’ discretion. Thirty-day major adverse cardiac event rates were 11.1% in the bivalirudin group and 8.9% in the UFH group (p = 0.56); the primary efficacy end point was reached mainly because of periprocedural myocardial infarction; 1 patient in the bivalirudin group had stent thrombosis. Occurrence of the primary safety end point was 1.5% in the bivalirudin group and 9.9% in the UFH group (p = 0.0001); this benefit was essentially driven by the prevention of entry-site hematomas >10 cm (0.5% vs 6.9%, p = 0.002). In conclusion, Anti-Thrombotic Strategy for Reduction of Myocardial Damage During Angioplasty–Bivalirudin vs Heparin (ARMYDA-7 BIVALVE) indicates that bivalirudin, compared with UFH, causes significantly lower bleeding and has a similar incidence of major adverse cardiac events in patients with older age, diabetes mellitus, or chronic renal failure who undergo PCI.
In large randomized trials, the use of bivalirudin, a direct thrombin inhibitor, has been associated with significant reductions of bleeding events compared to unfractionated heparin (UFH) in patients who undergo percutaneous coronary intervention (PCI) for a variety of clinical syndromes. In those studies, bivalirudin was compared to UFH plus the systematic use of glycoprotein (GP) IIb/IIIa inhibitors, so it was not possible to discriminate the relative contribution of UFH versus GP IIb/IIIa inhibitors in determining the excess bleeding observed with the latter strategy. An inverse relation between the prevention of bleeding complications by bivalirudin and the ability of the drug to provide ischemic myocardial protection has been postulated, and this might be clinically meaningful, especially in patients with high clinical risk profiles. Given the absence of randomized evidence, the aim of the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty–Bivalirudin vs Heparin (ARMYDA-7 BIVALVE) study was to evaluate the safety and efficacy of bivalirudin versus UFH (with the provisional use of GP IIb/IIIa inhibitors in the 2 arms) in selected PCI patients presenting with ≥1 of the following features of elevated bleeding risk: age >75 years, diabetes mellitus, and chronic renal failure.
Methods
ARMYDA-7 BIVALVE is a spontaneous, unfunded clinical trial performed at 2 Italian institutions (Campus Bio-Medico University of Rome, Rome, Italy and Vito Fazzi Hospital, Lecce, Italy). The design of the study is illustrated in Figure 1 . Inclusion criteria were patients with angiographically documented coronary artery disease suitable for PCI and ≥1 of the following: age >75 years, diabetes mellitus (defined according to American Diabetes Association criteria ), and chronic renal failure (defined as creatinine clearance of 30 to 60 ml/min). Creatinine clearance was calculated using the Cockcroft-Gault formula as [(140 − age) × weight/serum creatinine × 72], with female gender adjustment by multiplying creatinine clearance by 0.85. Exclusion criteria were primary PCI for acute myocardial infarction, bleeding diathesis or major bleeding <4 weeks, long-term warfarin therapy, platelet count <70 × 10 9 /L, and end-stage renal failure with creatinine clearance <30 ml/min. From June 1, 2009, to June 30, 2011, a total of 401 patients meeting the enrollment criteria were included; patients were randomly allocated to receive before the procedure UFH 75 IU/kg body weight (n = 203) or bivalirudin as a single bolus of 0.75 mg/kg followed by infusion during PCI of 1.75 mg/kg/hour (n = 198). Eligible patients were assigned to the allocation arm using an electronic spreadsheet indicating group assignment by random numbers. Study drug administration was performed on an open-label basis without blinding. GP IIb/IIIa inhibitors were used at the operator’s discretion in the 2 arms, especially in patients with high thrombotic burden at angiography and in those with slow-flow or no-reflow phenomenon. Routine measurement of activated clotting time during PCI was not performed. Interventions were performed using standard techniques. All patients were pretreated with aspirin (≥100 mg/day) and received a 600-mg loading dose of clopidogrel ≥6 hours before the procedure ; after PCI, aspirin (100 mg/day) was given indefinitely and clopidogrel (75 mg/day) for ≥1 month (12 months in those with acute coronary syndromes [ACS] or receiving drug-eluting stents), in all patients irrespective of the randomization assignment.
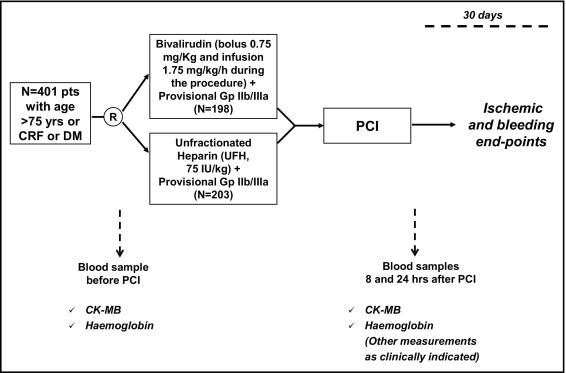
In all patients, blood samples were obtained before and at 8 and 24 hours after the intervention to measure hemoglobin, creatine kinase-MB (mass), and troponin I (mass) levels; further determinations were performed if clinically indicated. Creatine kinase-MB and troponin I measurements were done using the Access 2 immunochemiluminometric assay (Beckman Coulter, Brea, California); the upper limit of normal was defined as the 99th percentile of the normal population with a total imprecision of <10%, according to joint European Society of Cardiology and American College of Cardiology guidelines, and normal limits were ≤3.6 ng/ml for creatine kinase-MB and ≤0.034 ng/ml for troponin I.
Physicians performing laboratory testing as well as those adjudicating events during hospitalization were not aware of the randomization assignment; follow-up evaluation was obtained by office visit at 1 month, when hemoglobin levels were also measured and when a blinded investigator adjudicated events occurring from discharge to 30 days. Each patient gave informed consent to participate in the study. The study was approved by the institutional review boards of the institutions involved. The trial was not supported by any external source of funding.
The primary safety end point was the occurrence of any bleeding event or entry-site complications up to 1 month. Major bleeding was defined as intracranial bleeding or clinically overt bleeding associated with a decrease in hemoglobin of >5 g/dl, according to the Thrombolysis In Myocardial Infarction (TIMI) criteria ; minor bleeding was considered a clinically overt hemorrhage associated with a decrease in hemoglobin of 3 to 5 g/dl. Entry-site complications were hematoma >10 cm in diameter, pseudoaneurysm, and arteriovenous fistula. The arterial sheath was removed when the activated clotting time was <180 seconds; arteries were closed by manual compression until achievement of full hemostasis plus an additional 5 minutes. No vascular closure device was used.
The primary efficacy end point of the ARMYDA-7 BIVALVE trial was the 30-day incidence of major adverse cardiac events (cardiac death, myocardial infarction, target vessel revascularization, or definite or probable stent thrombosis). Stent thrombosis was defined according to the Academic Research Consortium. Periprocedural myocardial infarction was defined following these definitions according to pre-PCI clinical presentation: in patients with normal baseline creatine kinase-MB levels (i.e., receiving angioplasty for stable or unstable angina), it was defined as a postintervention increases of creatine kinase-MB >3 × the 99th percentile of the upper reference limit ; in patients with non–ST-segment elevation myocardial infarctions (i.e., with increased baseline creatine kinase-MB levels), a subsequent elevation ≥50% of the baseline creatine kinase-MB value was applied to detect periprocedural myocardial infarction. Target vessel revascularization included bypass surgery or repeat PCI of the target vessel(s).
Secondary end points included (1) any postprocedural increase of cardiac markers greater than the upper limit of normal (creatine kinase-MB and troponin I) and (2) evaluation of efficacy and safety outcomes in the 2 arms according to clinical presentation (i.e., stable angina vs non–ST-segment elevation ACS.
Hypothesizing a 10.5% overall rate of bleeding and entry-site complications at 1 month in the UFH arm, as observed in a previous study evaluating bivalirudin in patients with diabetes who underwent PCI, a study population of ≥397 patients was determined to provide 80% power to detect a 66% risk reduction in bleeding events in the bivalirudin arm at a 2-tailed α level of 0.05. Categorical variables are expressed as percentages and continuous variables as mean ± SD, unless otherwise specified. Proportions were compared using Fisher’s exact tests when the expected frequency was <5; otherwise, chi-square tests (with Yates’ correction) were used. Continuous variables between the 2 arms were compared using Student’s t tests for normally distributed values (as assessed by Kolmogorov-Smirnov test); otherwise, Mann-Whitney U tests were used. Event-free survival analysis was performed using the Kaplan–Meier method. All calculations were performed using SPSS version 15.0 (SPSS, Inc., Chicago, Illinois), and 2-sided p values <0.05 were considered significant.
Results
Clinical, angiographic, and procedural features were similar in the 2 arms and are listed in Tables 1 and 2 . In particular, the prevalence of patients aged >75 years was 38% in the bivalirudin group and 39% in the UFH group, diabetes mellitus was present in 67% and 59%, chronic renal failure was present in 22% and 20%, and non–ST-segment elevation ACS were present in 36% and 43%, respectively.
| Characteristic | Bivalirudin | UFH | p Value |
|---|---|---|---|
| (n = 198) | (n = 203) | ||
| Age (years) | 70.3 ± 8.4 | 70.1 ± 9.7 | 0.83 |
| Age >75 years | 76 (38%) | 80 (39%) | 0.91 |
| Men | 141 (71%) | 148 (72%) | 0.79 |
| Diabetes mellitus | 134 (67%) | 120 (59%) | 0.09 |
| Non-insulin-dependent | 98 (49%) | 80 (39%) | 0.05 |
| Insulin-dependent | 36 (18%) | 40 (20%) | 0.79 |
| Systemic hypertension | 178 (89%) | 187 (92%) | 0.55 |
| Current smoker | 35 (17%) | 27 (13%) | 0.28 |
| Previous myocardial infarction | 74 (37%) | 69 (34%) | 0.55 |
| Previous coronary revascularization | 85 (42%) | 94 (46%) | 0.50 |
| Chronic renal failure | 45 (22%) | 42 (20%) | 0.71 |
| Serum creatinine (mg/dl) | 1.04 ± 0.39 | 1.08 ± 0.78 | 0.48 |
| Clinical presentation | |||
| Non–ST-segment elevation myocardial infarction | 23 (12%) | 24 (13%) | 0.93 |
| Unstable angina pectoris | 38 (19%) | 30 (14%) | 0.30 |
| Stable angina pectoris | 137 (69%) | 149 (73%) | 0.41 |
| Left ventricular ejection fraction <50% | 28 (14%) | 38 (17%) | 0.27 |
| Statin use | 175 (88%) | 172 (84%) | 0.36 |
| Aspirin | 198 (100%) | 203 (100%) | — |
| Clopidogrel 600-mg loading dose | 198 (100%) | 198 (100%) | — |
| Characteristic | Bivalirudin | UFH | p Value |
|---|---|---|---|
| (n = 198) | (n = 203) | ||
| Site of access | |||
| Femoral | 193 (98%) | 199 (98%) | 0.97 |
| Radial | 5 (2%) | 4 (2%) | 0.97 |
| Target coronary vessel | |||
| Left anterior descending | 103 (44%) | 103 (44%) | 0.93 |
| Left circumflex | 69 (29%) | 67 (28%) | 0.92 |
| Right | 60 (25%) | 64 (27%) | 0.75 |
| Left main | 3 (1%) | 1 (0.4%) | 0.62 |
| Saphenous vein graft | 2 (1%) | 2 (1%) | 0.62 |
| Multivessel intervention | 37 (18%) | 32 (15%) | 0.52 |
| Stent diameter (mm) | 3.03 ± 0.49 | 2.99 ± 0.78 | 0.54 |
| Total stent length (mm) | 17.5 ± 5.3 | 17.1 ± 5.5 | 0.46 |
| Balloon only | 11 (5%) | 14 (6%) | 0.73 |
| Stents/patient | 1.43 ± 0.8 | 1.46 ± 0.9 | 0.70 |
| Use of drug-eluting stents | 54 (27%) | 58 (28%) | 0.86 |
| Use of GP IIb/IIIa inhibitors | 24 (12%) | 29 (14%) | 0.62 |
Most patients (98% in the 2 groups) received the intervention using the femoral approach. Procedural success was obtained in 196 of 198 patients (99%) in the bivalirudin group and 201 of 203 patients (99%) in the UFH group (p = 0.63); all unsuccessful procedures were due to failure to cross a chronic total occlusion. Use of GP IIb/IIIa inhibitors was 12% and 14%, respectively. No procedural side branch (≥2 mm) closure occurred. No patient required emergency coronary artery bypass surgery.
The primary safety end point occurred in 1.5% of patients in the bivalirudin arm (3 of 198) and 9.9% (20 of 203) of those in the UFH arm (odds ratio 0.14, 95% confidence interval 0.03 to 0.51, p = 0.0001; Figure 2 ). The incidence of this composite end point was driven mainly by entry-site hematomas >10 cm in the 2 groups (0.5% vs 6.9%, odds ratio 0.07, 95% confidence interval 0.01 to 0.50, p = 0.002). No patient had catheter thrombosis. Major bleedings were all gastrointestinal and occurred in 1 patient (0.5%) versus 2 (1%) (p = 0.98), respectively; 1 patient (0.5%) in the bivalirudin arm had a minor bleeding (urethral bleeding) compared to 4 in the UFH group (2%; gastrointestinal bleeding in 1 patient, gum bleeding in 1, and urethral bleeding in the remaining 2 patients) (p = 0.38). One patient in both arms was transfused.




