Long-term endurance sports are associated with atrial remodeling and an increased risk for atrial fibrillation (AF) and atrial flutter. Pro–atrial natriuretic peptide (pro-ANP) is a marker of atrial wall tension and elevated in patients with AF. The aim of this study was to test the hypothesis that atrial remodeling would be perpetuated by repetitive episodes of atrial stretching during strenuous competitions, reflected by elevated levels of pro-ANP. A cross-sectional study was performed on nonelite runners scheduled to participate in the 2010 Grand Prix of Bern, a 10-mile race. Four hundred ninety-two marathon and nonmarathon runners applied for participation, 70 were randomly selected, and 56 entered the final analysis. Subjects were stratified according to former marathon participations: a control group (nonmarathon runners, n = 22), group 1 (1 to 4 marathons, n = 16), and group 2 (≥5 marathons, n = 18). Results were adjusted for age, training years, and average weekly endurance training hours. The mean age was 42 ± 7 years. Compared to the control group, marathon runners in groups 1 and 2 had larger left atria (25 ± 6 vs 30 ± 6 vs 34 ± 7 ml/m 2 , p = 0.002) and larger right atria (27 ± 7 vs 31 ± 8 vs 35 ± 5 ml/m 2 , p = 0.024). Pro-ANP levels at baseline were higher in marathon runners (1.04 ± 0.38 vs 1.42 ± 0.74 vs 1.67 ± 0.69 nmol/L, p = 0.006). Pro-ANP increased significantly in all groups after the race. In multiple linear regression analysis, marathon participation was an independent predictor of left atrial (β = 0.427, p <0.001) and right atrial (β = 0.395, p = 0.006) remodeling. In conclusion, marathon running was associated with progressive left and right atrial remodeling, possibly induced by repetitive episodes of atrial stretching. The altered left and right atrial substrate may facilitate atrial arrhythmias.
There is a growing population of veteran endurance athletes regularly participating in training and competition. Recent data have documented an increased risk for atrial fibrillation (AF) and atrial flutter, especially in long-term endurance athletes. Lifetime training hours were associated with atrial enlargement in professional and nonelite athletes. Increased left atrial volume conferred a higher risk for AF in middle-aged marathon runners. Current sport practice and >1,500 lifetime training hours were associated with a higher risk for AF. Volume and pressure overload during exercise conditioning have been suggested as a possible mechanism of atrial remodeling. Pro–atrial natriuretic peptide (pro-ANP) is released in the atria by mechanical stretching. Pro-ANP levels are elevated in patients with paroxysmal AF and predict the risk for recurrent episodes of AF. Endurance training and competition differ with respect to exercise intensity. We hypothesized that atrial remodeling in athletes would be perpetuated by repetitive episodes of atrial stretching during strenuous competitions, reflected by elevated levels of pro-ANP at rest and after competition. The aim of this study was to demonstrate an independent effect of marathon running on left and right atrial remodeling.
Methods
The Grand Prix of Bern is among the most popular 10-mile races in Switzerland, with >25,000 participants. Marathon and nonmarathon runners were recruited by an open invitation letter published on the event’s Web page. All athletes applied via e-mail and provided age, endurance training years, average endurance and strength training hours per week, and number of marathon participations. Calculation of average training hours was based on the athletes’ estimations and/or exercise diaries. Measurement of training years started in adulthood (≥18 years). We included male athletes aged ≥30 years. We excluded subjects with hypertension (blood pressure >140/90 mm Hg), histories of cardiovascular disease (e.g., mitral regurgitation or diastolic dysfunction), and episodes of AF. Study participants were randomly selected and stratified into 3 groups according to former marathon participations: a control group (physically active nonmarathon runners), group 1 (1 to 4 marathons), and group 2 (≥5 marathons). Group thresholds were determined after subject selection to ensure equal group sizes. Baseline examination included electrocardiography, blood sampling of pro-ANP, echocardiography, 24-hour Holter monitoring, and cardiopulmonary exercise testing on a treadmill. Runners were examined 12 to 2 weeks (mean 6 ± 3) before the race. Blood sampling of pro-ANP was repeated immediately (<15 minutes) after the 10-mile race. Two experienced cardiologists blinded to the athletes’ performance and marathon participation status performed all analyses. All athletes provided written informed consent, and the protocol was approved by the local ethics committee.
Blood was collected into lithium-heparin tubes, immediately centrifuged, and frozen. Samples were stored at −80°C until analysis. The plasma concentration of pro-ANP was measured using a commercially available enzyme immunoassay (Biomedica, Vienna, Austria).
Standard transthoracic echocardiography was performed according to the recommendations of the European Association of Echocardiography (iE33 with an S5-1 2.5-MHz transducer; Phillips Healthcare, Zurich, Switzerland). Images were stored digitally and analyzed off-line. Left and right atrial and left ventricular (LV) end-diastolic volumes were calculated according to current recommendations and indexed for body surface area. The LV ejection fraction was derived from the end-diastolic and end-systolic volumes. Pulsed-wave Doppler was performed in the apical 4-chamber view to obtain peak early filling (E-wave) and late diastolic filling (A-wave) velocities, the E/A ratio, the deceleration time of the early filling wave, and the isovolumic relaxation time. Pulsed-wave tissue Doppler imaging was performed in the apical 4-chamber view to acquire peak septal mitral annular velocity (e′ and a′).
Twelve-lead rest electrocardiography was performed with the athlete in the supine position. Heart rate and blood pressure at rest were determined after 5 minutes. Spiroergometric testing was performed on a treadmill according to the recommendations of the American Heart Association. We used a ramp protocol starting at 7.2 km/hour, with speed increasing by 0.2 km/hour every 20 seconds until exhaustion. Athletes were encouraged to reach a respiratory exchange ratio of ≥1.05. Respiratory parameters were measured continuously in an open spirometric system (CS 200; Schiller-Reomed AG, Dietikon, Switzerland) and registered as averaged values over 30 seconds. Blood pressure was measured at peak exercise. The aerobic and anaerobic thresholds were determined according to current recommendations.
Twenty-four-hour Holter monitoring was performed before the race. Three-channel electrocardiograms were recorded using a Lifecard CF digital recorder (Spacelabs Healthcare, Nuremberg, Germany) and manually analyzed and interpreted using Pathfinder software. Premature atrial and ventricular contractions were classified according to onset and QRS morphology.
Data were analyzed using SPSS for Windows version 17.0 (SPSS, Inc., Chicago, Illinois). The normality of quantitative variables was verified using the Kolmogorov-Smirnov test. The 3 groups were compared using analysis of variance. Additionally, analysis of covariance was used for adjustment for age, training years, and average weekly training hours. Multiple linear regression analysis was performed to analyze the impacts of age, training years, average weekly endurance training hours, and number of marathon participations on left and right atrial remodeling. For correlations of left and right atrial volumes with pro-ANP, Pearson’s correlation coefficient was calculated. A 2-sided p value <0.05 was considered to indicate statistical significance.
Results
Four hundred ninety-two male athletes applied for participation, and 70 were randomly selected. Fourteen runners had to be excluded (7 did not participate in the race, 4 had histories of AF, 2 had undiagnosed arterial hypertension with diastolic dysfunction, and 1 had mitral valve prolapse). Fifty-six runners entered the final analysis. The mean age was 42 ± 7 years. Marathon runners in group 2 showed a tendency to be older. Compared to the control group, marathon runners had significantly more training years and average weekly endurance training hours and had participated more often in 10-mile races. Athletes showed no differences in body composition and average weekly strength training hours ( Table 1 ).
| Variable | Marathon Participations | p Value | ||
|---|---|---|---|---|
| Control Group (0) | Group 1 (1–4) | Group 2 (≥5) | ||
| (n = 22) | (n = 16) | (n = 18) | ||
| Age (years) | 40 ± 6 | 40 ± 6 | 45 ± 8 | 0.054 |
| Body mass index (kg/m 2 ) | 23.7 ± 2.1 | 23.3 ± 1.7 | 23.2 ± 1.8 | 0.635 |
| Body surface area (m 2 ) | 1.92 ± 0.12 | 1.95 ± 0.12 | 1.94 ± 0.14 | 0.740 |
| Marathon participations | — | 2.2 ± 1.3 | 11.8 ± 7.5 | <0.001 |
| 10-mile race participations | 3.2 ± 5.0 | 5.8 ± 4.5 | 9.3 ± 9.3 | 0.004 |
| Endurance training years | 10.3 ± 9.2 | 13.7 ± 6.1 | 18.9 ± 8.1 | 0.006 |
| Endurance training (hours/week) | 3.5 ± 2.8 | 4.9 ± 3.3 | 6.5 ± 4.2 | 0.034 |
| Strength training (hours/week) | 0.6 ± 0.7 | 0.7 ± 0.5 | 0.8 ± 1.0 | 0.669 |
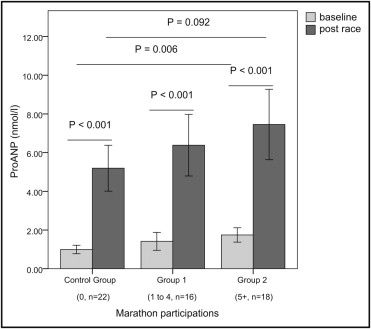
Marathon runners in group 2 had significantly higher baseline levels of pro-ANP, compared to those in group 1 and the control group. Pro-ANP increased significantly in every group after the race. Peak levels of pro-ANP showed a tendency toward higher values in marathon runners in group 2 ( Figure 1 ) . Ten-mile race times were significantly shorter and oxygen uptake at the aerobic and anaerobic thresholds was significantly higher in marathon runners, but the effect was lost when adjusted for age, training years, and training hours. Heart rate at rest was lower in marathon runners, while peak heart rate, blood pressure at rest and peak exercise, and peak oxygen uptake showed no significant differences between groups ( Table 2 ).
| Variable | Marathon Participations | p Value | |||
|---|---|---|---|---|---|
| Control Group (0)(n = 22) | Group 1 (1–4)(n = 16) | Group 2 (≥5)(n = 18) | Unadjusted | Adjusted ⁎ | |
| Competition | |||||
| 10-mile race time | 84:58 ± 09:35 | 76:21 ± 10:40 | 77:23 ± 12:14 | 0.048 | 0.193 |
| Exercise testing | |||||
| Heart rate at rest (beats/min) | 61 ± 8 | 51 ± 8 | 51 ± 8 | <0.001 | 0.006 |
| Peak heart rate (beats/min) | 181 ± 7 | 180 ± 10 | 178 ± 10 | 0.558 | 0.765 |
| Systolic BP at rest (mm Hg) | 124 ± 7 | 120 ± 8 | 124 ± 11 | 0.321 | 0.326 |
| Peak systolic BP (mm Hg) | 180 ± 15 | 181 ± 16 | 180 ± 15 | 0.977 | 0.658 |
| V o 2 aerobic threshold (ml/min/kg) | 25.1 ± 4.9 | 28.3 ± 4.1 | 29.3 ± 5.2 | 0.026 | 0.157 |
| V o 2 anaerobic threshold (ml/min/kg) | 41.8 ± 7.1 | 46.4 ± 6.5 | 48.2 ± 6.8 | 0.012 | 0.109 |
| V o 2 peak (ml/min/kg) | 50.9 ± 6.2 | 53.5 ± 7.3 | 55.0 ± 6.3 | 0.143 | 0.691 |
| Echocardiography | |||||
| Left atrial volume index (ml/m 2 ) | 25 ± 6 | 30 ± 6 | 34 ± 7 | <0.001 | 0.002 |
| Right atrial volume index (ml/m 2 ) | 27 ± 7 | 31 ± 8 | 35 ± 5 | 0.002 | 0.024 |
| Right atrial volume/left atrial volume ratio | 1.14 ± 0.33 | 1.08 ± 0.31 | 1.07 ± 0.24 | 0.699 | 0.650 |
| LV end-diastolic volume (ml/m 2 ) | 52 ± 9 | 56 ± 8 | 55 ± 11 | 0.297 | 0.433 |
| LV mass (g/m 2 ) | 98 ± 12 | 110 ± 18 | 115 ± 17 | 0.002 | 0.014 |
| Relative wall thickness † | 0.43 ± 0.06 | 0.41 ± 0.05 | 0.44 ± 0.06 | 0.267 | 0.744 |
| LV ejection fraction (%) | 64 ± 4 | 63 ± 4 | 64 ± 5 | 0.706 | 0.555 |
| Peak E (cm/s) | 78.5 ± 10.4 | 89.7 ± 15.9 | 78.0 ± 14.4 | 0.022 | 0.019 |
| Peak A (cm/s) | 53.5 ± 9.5 | 54.6 ± 9.5 | 55.5 ± 12.1 | 0.831 | 0.464 |
| E/A | 1.5 ± 0.3 | 1.7 ± 0.3 | 1.4 ± 0.3 | 0.111 | 0.106 |
| Peak e′ septal (cm/s) | 11.0 ± 1.6 | 11.2 ± 1.0 | 10.8 ± 1.8 | 0.758 | 0.868 |
| Peak a′ septal (cm/s) | 9.2 ± 1.2 | 8.2 ± 0.9 | 8.5 ± 1.2 | 0.027 | 0.062 |
| Isovolumic relaxation time (ms) | 89 ± 13 | 83 ± 11 | 89 ± 17 | 0.412 | 0.388 |
| E wave deceleration time (ms) | 189 ± 29 | 173 ± 28 | 181 ± 33 | 0.254 | 0.207 |
| 24-hour Holter monitoring | |||||
| Minimal heart rate (beats/min) | 46 ± 6 | 42 ± 5 | 41 ± 5 | 0.008 | 0.087 |
| Premature atrial contractions | 4 ± 4 | 7 ± 7 | 11 ± 7 | 0.002 | 0.040 |
| Premature ventricular contractions | 13 ± 30 | 7 ± 16 | 143 ± 533 | 0.328 | 0.372 |
⁎ Adjusted for age, training years, and average weekly endurance training hours.
† Calculated as (interventricular septal thickness + LV posterior wall thickness)/LV end-diastolic diameter.
Left and right atrial volume indexes increased significantly with marathon participations, while the right to left atrial volume ratio remained stable ( Figure 2 , Table 2 ). We found a significant correlation of left and right atrial volumes (r = 0.497, p <0.001). Left atrial enlargement (>29 ml/m 2 ) was present in 5 of 22 athletes (22.7%) in the control group, 10 of 16 athletes (62.5%) in group 1, and 14 of 18 athletes (77.8%) in group 2 (p <0.001). LV mass index increased significantly with the number of marathon participations, while relative wall thickness remained stable. Marathon and nonmarathon runners showed no relevant differences in LV end-diastolic volume and parameters of systolic and diastolic LV function ( Table 2 ).





