Drug-eluting stents have been effective in randomized controlled trials, but their safety and efficacy in patients with insulin-dependent diabetes has not been well studied. Baseline clinical and angiographic characteristics and in-hospital and follow-up events were recorded for enrolled patients. From October 2005 and October 2006, 581 patients with insulin-dependent diabetes and 1,078 with non-insulin-dependent diabetes treated with sirolimus- and paclitaxel-eluting stents were enrolled at 98 sites. The composite of death, myocardial infarction, and stroke, defined as major adverse cardiac and cerebrovascular events, as well as target vessel revascularization was used as the primary end point. Multiple logistic regression analysis was used to adjust for confounding parameters. Baseline clinical characteristics were more severe in patients with insulin-dependent diabetes, whereas descriptive characteristics were not unique. At 1-year follow-up, the comparison between the 2 groups revealed significantly higher rates of overall death (7.4% vs 4.6%, p <0.05), target vessel revascularization (15.1% vs 10.4%, p <0.05), and overall stent thrombosis (6.5% vs 4.1%, p <0.05) for insulin-dependent patients, while rates of major adverse cardiac and cerebrovascular events were not significantly different (12.8% vs 9.9%, p = 0.09). These results persisted even after risk adjustment for heterogenous baseline characteristics of the 2 groups. In conclusion, the data generated from the German Drug-Eluting Stent (DES.DE) registry revealed that even with drug-eluting stents, the annual risk for death, target vessel revascularization, and thrombotic events remains higher in patients with insulin-dependent diabetes compared to those with non-insulin-dependent diabetes.
Drug-eluting stents (DES) are established for the treatment of coronary artery lesions in patients with diabetes, but the differential safety and efficacy of DES in patients with insulin-dependent diabetes mellitus (IDDM) and those with non-insulin-dependent diabetes mellitus (NIDDM) is unknown, because all randomized controlled trials have been underpowered to address this question, and current conclusions rely only on subgroup analyses. In the present study, we compared clinical outcomes after DES implantation between patients with IDDM and those with NIDDM followed over 1 year in a “real-world” setting.
Methods
The large, prospective, multicenter German Drug-Eluting Stent (DES.DE) registry design has been previously published. Briefly, the registry was initiated in October 2005 as an observational cohort study by Deutsche Gesellschaft für Kardiologie (the German Cardiac Society), Bundesverband Niedergelassener Kardiologen (the German Society of Cardiologists in Private Practice), and Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (Working Group of Hospital Cardiologists) to evaluate the therapeutic benefit of DES under real-world conditions of the German health system. The use of DES had to meet certain quality criteria specified and confirmed by the DES.DE Steering Committee and adopted from the European Society of Cardiology and American College of Cardiology guidelines for percutaneous coronary intervention. In phase I of the registry (October 2005 to October 2006), only the 2 United States Food and Drug Administration–approved DES, the Taxus paclitaxel-eluting stent (Boston Scientific Corporation, Natick, Massachusetts) and the Cypher sirolimus-eluting stent (Cordis Corporation, Miami Lakes, Florida), met the quality criteria of the registry. In all cases in this registry, the interventional strategy, including the choice of stent, the use of intravascular ultrasonography, and the choice of periprocedural adjunctive therapy, was at the discretion of the responsible physician regardless of clinical setting.
Data were collected via Internet platform by Institut für Klinische Kardiovaskuläre Forschung (the Institute for Clinical Cardiovascular Research) of the German Cardiac Society. The European Cardiology Audit and Registration Standard was adapted for patient and lesion data. Written informed consent for processing anonymous data at Institut für Herzinfarktforschung (the Heart Center Ludwigshafen) and Institut für Klinische Kardiovaskuläre Forschung was obtained. Baseline clinical and angiographic characteristics, which were assessed by automated standard quantitative coronary angiographic software at each participating center, and procedural and clinical in-hospital events were recorded for all enrolled patients. Paper-based clinical follow-up assessments as well as external telephone surveys were performed 3, 6, 9, and 12 months after the initial stent placement and are planned for 5-year follow-up. Relevant events and the adjudication of stent thrombosis as well as randomly selected angiograms were forwarded to 2 independent critical event committees for adjudication and validity.
Patients with diabetes were identified when receiving treatment with insulin, an oral antidiabetic agent, or dietary therapy for abnormal glucose levels according to World Health Organization criteria ; before percutaneous coronary intervention, all patients had undergone specific evaluation of fasting glucose levels without insulin or oral drugs at the outpatient diabetes clinic and were divided according to their use of insulin. The primary end points of the present survey of patients in DES.DE were prespecified as occurrence rates of target vessel revascularization (TVR) and major adverse cardiac and cerebrovascular events (MACCEs; defined as the composite of death [cardiac and noncardiac], myocardial infarction, and stroke). Myocardial infarction was defined as ST-segment elevation myocardial infarction (ST-segment elevation ≥1 mm in ≥2 standard leads or ≥2 mm in ≥2 contiguous precordial leads or development of new left bundle branch block) or as non–ST-segment elevation myocardial infarction (pathologic increases in cardiac-specific enzymes, with creatine kinase-MB >1.5 times normal limits or troponin T or troponin I >99th percentile of normal value). TVR included repeat procedures, either percutaneous coronary intervention or coronary artery bypass grafting, in the target vessel. The definition of major adverse cardiac events may slightly vary among clinical studies. In a number of major adverse cardiac event definitions, different types of death (either cardiac or total death rate) and revascularization parameters such as TVR have been used. Because the use of different definitions of major adverse cardiac events can cause confusion when comparing rates among trials, the Steering Committee decided to use only MACCEs as defined previously. Chronic kidney disease was assumed if the patient had a serum creatinine level ≥1.3 mg/dl or had ever required temporary or ongoing maintenance hemodialysis, and dyslipidemia was assumed if low-density lipoprotein cholesterol and triglycerides were >200 mg/dl and high-density lipoprotein cholesterol was <40 mg/dl. Patients in the DES.DE registry were discouraged from undergoing routine angiography, so all reinterventions were clinically driven. Stent thrombosis was classified according to definitions proposed by the Academic Research Consortium.
Statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina). Demographic characteristics, preexisting risk factors, procedure-related variables, and 1-year outcomes were summarized using means and SDs for continuous variables and frequencies and percentages for categorical variables. Differences in baseline, procedural, and angiographic characteristics and in-hospital and follow-up data were compared between patients with IDDM and those with NIDDM using chi-square tests, while continuous variables were compared using 2-tailed Wilcoxon’s rank-sum tests. The Cochran-Armitage test was used to analyze trends over time. Two-tailed p values <0.05 were considered significant. One-year event-free survival for the composite of myocardial infarction and stroke as well as for TVR was demonstrated using Kaplan-Meier curves. The effect of IDDM on clinical events during the follow-up period was evaluated using multiple logistic regression with backward selection (α = 0.05) to adjust for confounding parameters. A multivariate logistic regression model was developed with covariates associated with IDDM in univariate analysis using a p value <0.25. Moreover, the following variables were entered into the multivariate model with MACCEs as the end point: insulin treatment, impaired left ventricular ejection fraction (<40%), active smoking, ST-segment elevation myocardial infarction, non–ST-segment elevation myocardial infarction, unstable angina, age >75 years, female gender, body mass index >25 kg/m 2 , hypercholesterolemia, congestive heart failure, previous myocardial infarction, previous percutaneous coronary intervention, previous coronary artery bypass grafting, previous stroke, triple-vessel disease, and type C stenosis. In the second multivariate model, with TVR as the end point, all variables from model 1 and additional variables, such as the use of sirolimus-eluting stents, bypass graft intervention, chronic total occlusion recanalization, in-stent restenosis, maximal length of stents, and smallest diameter of stents, were entered. Because there were few events during 1-year follow-up, these multivariate analyses were restricted to the primary end points of MACCEs and TVR.
Results
From October 2005 to October 2006, 1,659 patients with diabetes treated with either paclitaxel-eluting stents or sirolimus-eluting stents were enrolled at 98 sites. The analysis was based on 962 patients (58.0%) receiving only paclitaxel-eluting stents, 668 (40.3%) receiving only sirolimus-eluting stents, and 29 (1.7%) who received a combination of paclitaxel-eluting stents and sirolimus-eluting stents. Among these patients, 581 (35.0%) had IDDM and 1,078 had NIDDM, with 849 (78.7%) receiving oral antidiabetic agents and 229 (21.3%) managed by antidiabetic diet and physical activity only. A male predominance was found in patients with NIDDM (75.0% vs 65.4% in IDDM, p <0.0001) subjected to DES. Moreover, patients with IDDM had a higher mean body mass index (29.3 vs 28.5 kg/m 2 in NIDDM, p <0.01), more often had chronic kidney disease (24.9% vs 13.0% in NIDDM, p <0.0001), and more often had severely impaired left ventricular function (7.2% vs 3.9%, p <0.05) ( Table 1 ).
| Variable | IDDM | NIDDM | p Value |
|---|---|---|---|
| (n = 581) | (n = 1,078) | ||
| Men | 65.4% | 75.0% | <0.0001 |
| Age (years) (mean ± SD) | 66.9 ± 9.4 | 66.6 ± 9.4 | 0.41 |
| Body mass index (kg/m 2 ) | 29.3 | 28.5 | <0.01 |
| Diabetes mellitus | |||
| Only dietary control | 0% | 21.3% | |
| Oral hypoglycemic agents | 37.6% | 78.7% | |
| Insulin | 100% | 0% | |
| Dyslipidemia | 80.7% | 83.5% | 0.16 |
| Chronic kidney disease | 24.9% | 13.0% | <0.0001 |
| History of heart failure | 22.0% | 19.0% | 0.17 |
| Hypertension | 92.4% | 92.6% | 0.87 |
| Atrial fibrillation | 11.2% | 10.2% | 0.54 |
| Smoking | |||
| Current | 14.9% | 19.3% | <0.05 |
| Previous | 54.4% | 55.1% | 0.80 |
| Family history of coronary artery disease | 32.1% | 31.1% | 0.76 |
| Previous myocardial infarction | 34.1% | 30.2% | 0.11 |
| Previous known percutaneous coronary intervention | 48.2% | 43.3% | 0.06 |
| Previous known coronary artery bypass grafting | 18.0% | 14.4% | 0.05 |
| Previous stroke | 7.4% | 5.3% | 0.09 |
| Ejection fraction | |||
| >50% | 62.2% | 66.1% | <0.05 |
| 41%–50% | 21.0% | 19.3% | |
| 31%–40% | 9.6% | 10.6% | |
| <30% | 7.2% | 3.9% |
Approximately 1/3 of the patients in the 2 groups were admitted with acute coronary syndromes, and nearly half of the patients had triple-vessel disease, reflecting all comers ( Table 2 ). A total of 2,295 stents were implanted for 1,897 lesions in 1,659 patients (1.34 per patient and 1.21 per lesion), with a procedural success rate of 97.7%. Stents were deployed in 100% of percutaneous coronary intervention cases, but in 1.3%, patients’ authorization for the use of data was revoked. As a result, outcome data were authorized by 98.7% of all patients. The numbers of DES per patient and per lesion were equally distributed, with 1.35 stents/patient and 1.11 stents/lesion in patients with IDDM and 1.40 stents/patient and 1.16 stents/lesion in those with NIDDM. Direct stenting was performed in <1/2 of the patients (37.9% and 40.6%), with no significant difference between groups. DES used in patients with IDDM were usually longer (20 ± 7 vs 19 ± 7 mm, p <0.01) and smaller (2.9 ± 0.4 vs 3.0 ± 0.4 mm, p <0.0001) than those in patients with NIDDM ( Table 3 ).
| Variable | IDDM | NIDDM | p Value |
|---|---|---|---|
| Number of coronary arteries narrowed | |||
| 1 | 18.1% | 22.8% | <0.05 |
| 2 | 34.7% | 32.7% | |
| 3 | 47.2% | 44.5% | |
| Target coronary artery | |||
| Left anterior descending | 43.9% | 46.2% | 0.33 |
| Left circumflex | 27.4% | 22.5% | |
| Right | 26.0% | 29.3% | |
| Left main | 2.8% | 2.1% | |
| Bypass graft | 5.4% | 5.3% | |
| AHA/ACC lesion score | |||
| A | 10.2% | 12.2% | <0.01 |
| B | 65.4% | 57.3% | |
| C | 24.4% | 30.5% | |
| Acute coronary syndromes | |||
| STEMI | 10.2% | 12.1% | <0.05 |
| NSTEMI | 14.0% | 14.3% | |
| Unstable angina pectoris | 13.5% | 15.6% | |
| Elevated cardiac markers | 27.8% | 27.2% | |
| TIMI flow grade | |||
| 0 | 8.9% | 12.3% | <0.05 |
| 1 | 9.8% | 8.9% | |
| 2 | 23.6% | 19.9% | |
| 3 | 57.7% | 58.9% | |
| Chronic total occlusion | 2.9% | 3.7% | 0.41 |
| In-stent restenosis | 13.5% | 14.4% | 0.58 |
| Bifurcation | 12.9% | 15.4% | 0.15 |
| Variable | IDDM | NIDDM | p Value |
|---|---|---|---|
| Stenosis degree (%) | 85.2 ± 11.0 | 87.2 ± 11.2 | <0.0001 |
| Lesion diameter (mm) | 2.8 ± 0.4 | 2.9 ± 0.4 | <0.05 |
| Lesion length (mm) | 18 ± 9 | 18 ± 10 | 0.67 |
| Stent diameter (mm) | 2.9 ± 0.4 | 3.0 ± 0.4 | <0.0001 |
| Stent length (mm) | 20 ± 7 | 19 ± 7 | <0.01 |
| Paclitaxel-eluting stent | 62.0% | 55.6% | <0.01 |
| Sirolimuseluting stent | 36.7% | 42.2% | <0.05 |
| PES and SES | 1.3% | 2.2% | 0.22 |
| Device use | |||
| Intravascular ultrasonography | 0.8% | 0.4% | 0.28 |
| Rotablation | 0.8% | 0.3% | 0.17 |
| Cutting balloon | 0.2% | 0.4% | 0.37 |
| Direct stenting | 37.9% | 40.6% | 0.22 |
| Postprocedural TIMI flow grade 3 | 97.1% | 98.1% | 0.21 |
| Lesion complication | |||
| Abrupt closure | 0.2% | 0.3% | 0.50 |
| Side branch occlusion | 0.3% | 0.2% | 0.78 |
| Persistent flow reduction | 0.2% | 0% | 0.17 |
| Clopidogrel loading dose (mg) | |||
| 300 | 37.1% | 34.8% | 0.76 |
| 600 | 47.3% | 51.8% | 0.22 |
| GP IIb/IIIA antagonist | 15.2% | 16.8% | 0.39 |
The overall in-hospital MACCE rate was 2.4% in patients with IDDM and 2.2% in those with NIDDM (p = 0.81). Likewise, rates of postprocedural myocardial infarction, death, stroke, urgent revascularization, and severe bleeding complications were low, with no significant difference between groups ( Table 4 ). Conversely, rates of postinterventional renal failure (3.0% vs 1.2%, p <0.05) and hospital stay >3 days (35.5% vs 29%, p <0.01) were higher in the IDDM group. Antithrombotic medication at discharge was similar, using aspirin in 97.8%, clopidogrel in 99%, and a combination of dual-antiplatelet therapy with oral anticoagulation therapy in 3.0%.
| Variable | IDDM | NIDDM | p Value |
|---|---|---|---|
| In-hospital follow-up | |||
| All-cause death | 0.7% | 0.9% | 0.61 |
| Myocardial infarction | 1.2% | 1.1% | 0.87 |
| Stroke | 0.5% | 0.6% | 0.92 |
| MACCEs | 2.4% | 2.2% | 0.81 |
| Repeat urgent revascularization | |||
| Percutaneous coronary intervention | 0.5% | 0.6% | 0.92 |
| Coronary artery bypass grafting | 0.2% | 0.2% | 0.95 |
| Repeat elective revascularization | |||
| Percutaneous coronary intervention | 3.3% | 2.9% | 0.65 |
| Coronary artery bypass grafting | 0.5% | 0.6% | 0.74 |
| Renal failure | 3.0% | 1.2% | <0.05 |
| Severe bleeding complications | 0.5% | 0.8% | 0.59 |
| Hospitalization >3 days | 35.5% | 29.0% | <0.01 |
| Aspirin + clopidogrel + oral anticoagulation | 2.8% | 3.2% | 0.65 |
| 1-year follow-up | |||
| Clinical follow-up | 90.8% | 91.8% | 0.51 |
| All-cause death | 7.4% | 4.6% | <0.05 |
| Myocardial infarction | 4.9% | 3.7% | 0.27 |
| Stroke | 1.4% | 1.8% | 0.61 |
| MACCEs | 12.8% | 9.9% | 0.09 |
| TVR | 15.1% | 10.4% | <0.05 |
| Overall stent thrombosis | 6.5% | 4.1% | <0.05 |
| Definite | 0.6% | 0.7% | 0.76 |
| Aspirin | 93.0% | 94.6% | 0.22 |
| Clopidogrel | 58.8% | 55.3% | 0.24 |
| Oral anticoagulation | 9.9% | 9.2% | 0.72 |
| Bleeding | |||
| Major | 1.1% | 1.7% | 0.40 |
| Minor | 41.6% | 39.5% | 0.44 |
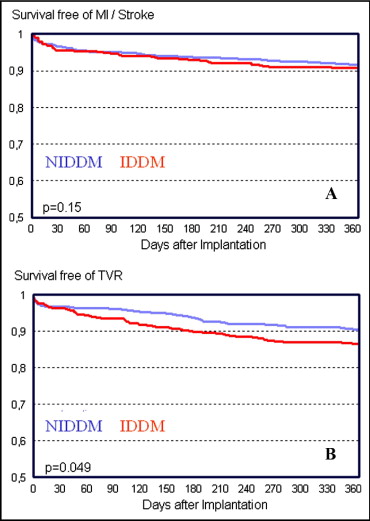
Clinical outcomes after a mean follow-up period of 12.4 months were assessed in 90.8% of patients with IDDM and 91.8% of those with NIDDM (p = 0.51; Table 4 ). No significant differences were noted between the groups with respect to the incidence of MACCEs (12.8% vs 9.9%, odds ratio [OR] 1.29, 95% confidence interval [CI] 0.95 to 1.75, p = 0.09). Compared to patients with NIDDM, those with IDDM had significantly higher rates of all-cause mortality (7.4% vs 4.6%, OR 1.67, 95% CI 1.07 to 2.60, p <0.05) and TVR (15.1% vs 10.4%, OR 1.29, 95% CI 0.95 to 1.75, p <0.05; Figure 1 ). A subgroup analysis comparing outcomes after using sirolimus-eluting stents or paclitaxel-eluting stents revealed no differences between the 2 DES in the overall diabetic population for the primary end points of MACCEs (sirolimus-eluting stents: OR 1.154, 95% CI 0.784 to 1.782) and TVR (sirolimus-eluting stents: OR 0.909, 95% CI 0.608 to 1.359), regardless of the antidiabetic treatment strategy ( Table 5 ). Because of the differences in baseline characteristics, a multivariate analysis was performed to determine the impact of insulin treatment on primary end points at 1 year. After risk adjustment, rates for MACCEs (OR 1.35, 95% CI 0.88 to 2.05, p <0.05) remained similar between patients with IDDM and those with NIDDM, while rates for TVR remained different between the 2 groups (OR 1.70, 95% CI 1.32 to 2.46, p <0.05). Rates of overall stent thrombosis were in the expected range and higher in patients with IDDM (6.5% vs 4.1%, OR 1.63, 95% CI 1.02 to 2.61, p <0.05). Aspirin, clopidogrel, and oral anticoagulation were used in 94.1%, 56.5%, and 9.5%, respectively, with no differences between groups.