The impact of diabetes mellitus (DM) on the outcome of patients requiring cardiac surgery has been investigated in previous decades. However, the profile of cardiac surgical practice is changing in addition to changes in patients’ risk profile, making the results inconclusive. In this study we sought to investigate the impact of DM on operative mortality and morbidity in patients undergoing cardiac surgery and adjust for patient and disease characteristics. In total 10,709 patients (9,229 nondiabetics and 1,480 diabetics) were admitted to the study; 5,557 patients (1,012 diabetics) underwent an isolated coronary operation, 1,775 patients (278 diabetics) underwent coronary plus valve operations, and 3,337 patients (209 diabetics) underwent valve operations. To control for differences in patient and disease characteristics, a propensity score (for DM) was performed. DM increased crude morbidity and this difference was maintained after risk adjustment for propensity score; conversely, the crude operative mortality risk was higher in diabetics but not significantly after adjustment for propensity score. Thereafter, DM remained independently associated to operative mortality risk in the valve population only (odds ratio 2.53, 95% confidence interval 1.45 to 4.4, p = 0.001). In conclusion, DM has a significant impact on operative mortality of patients undergoing heart valve surgery. Although diabetic patients undergoing coronary operations are not at increased risk of operative mortality, morbidity is significantly affected in the overall population.
The global burden of diabetes mellitus (DM) is rapidly increasing and approximately 8% of adults in developed countries have DM. Because of the proportion, it is expected that an increasing number of patients with DM will undergo cardiac surgery in the future, making diabetes-related operative risk assessment an important tool. The impact of DM on the outcome of patients requiring coronary operations has been systematically investigated in previous decades. However, results have been changing over time from series where DM was found to be associated with increased early and 30-day mortalities to more recent studies where this finding was not confirmed. More recently, because the profile of cardiac surgical practice is changing (coronary surgery is decreasing, whereas the number of patients requiring valve surgery is increasing) in addition to changes in patients’ risk profile, more attention has been placed on risk assessment of patients undergoing heart valve surgery. However, currently few risk models addressing this important issue have been proposed and, most important, these still have strict limitations that can lead to an underestimation of the weight of DM in risk stratification. In this study we sought to investigate the impact of DM on operative mortality and morbidity in a single large institutional series of patients undergoing cardiac surgery and consider patient and disease characteristics with propensity-score adjustment.
Methods
This is a retrospective study based on our prospective institutional database of cardiac surgical patients. The local ethics committee approved the study design and waived the need for an informed consent of patients. All patients provided written consent to the scientific treatment of their data in an anonymous form at time of hospitalization.
All patients operated from April 2000 to April 2009 were admitted to this study. Patients were assigned to the diabetic or nondiabetic group according to their condition at hospital admission. Exclusion criteria were age <18 years and congenital heart operations.
The study population included 10,709 patients; 5,557 patients underwent an isolated coronary operation, 1,775 patients underwent coronary plus valve operations, and 3,337 underwent valve operations (single or multiple, including valve plus ascending aorta operations).
Patients with DM were identified as those receiving oral antidiabetic treatment and/or insulin at time of surgery. Patients receiving nutritional modifications as the sole treatment for hyperglycemia were not considered diabetic; patients in whom DM was discovered and treated during hospitalization were considered diabetics. Insulin treatment of transient postoperative hyperglycemia was not considered a criterion for being included in the diabetic group. During and after the operation, blood glucose levels were controlled by insulin infusion to maintain a level <180 mg/dl. At the time of surgery, all patients were on state-of-the-art optimized medical therapy. Mean duration of DM was not available, and glucose tolerance, insulin resistance, and glycosylated hemoglobin levels were not evaluated.
Demographic (age, gender, and weight) data were collected. Preoperative laboratory assays included serum creatinine value (milligrams per deciliter) and hematocrit (percentage). Cardiac function was assessed by left ventricular ejection fraction measured before the operation with echocardiographic assessment. For repeated different measurements, the lowest value was used; other cardiac-related factors collected were recent (within 30 days) myocardial infarction, unstable angina, congestive heart failure (HF), preoperative use of intra-aortic balloon pump, and presence of active endocarditis. The following co-morbid conditions were recorded: chronic obstructive pulmonary disease treated with medication at time of surgery, previous cerebrovascular accident, chronic renal failure on dialytic treatment, previous vascular surgery, and previous cardiac surgery.
Operative data recorded were isolated coronary artery bypass graft (CABG) operation, CABG plus valve operation, valve operation, urgent operation, and cardiopulmonary bypass (CPB) duration (minutes).
Postoperative outcome data included mechanical ventilation time (hours), intensive care unit stay (days), postoperative hospital stay (days), acute renal failure (peak postoperative serum creatinine level >2.0 mg/dl and ≥2 times the preoperative value), sepsis, stroke, and surgical re-exploration. Major morbidity was defined according to the STS National Database Risk Stratification Subcommittee as 1 of the following: mechanical ventilation time >48 hours, sepsis or mediastinitis, acute renal failure, stroke, or surgical re-exploration. Myocardial infarction and acute respiratory insufficiency were separately considered additional outcome variables. Operative mortality was defined as in-hospital mortality or mortality within 30 days from the operation.
Data in text and tables are expressed as mean ± SD of the mean for continuous variables and as number and percentage for categorical variables. Differences between means were assessed using unpaired Student’s t test and Pearson chi-square test for differences between percentages. A propensity-score analysis determining the propensity of belonging to the diabetic group was performed with the following steps: (1) factors significantly different between diabetics and nondiabetics were established as previously stated, (2) factors associated to diabetic conditions (p <0.1) were entered into a multivariable stepwise forward logistic regression analysis, (3) the logistic equation provided a propensity score for likelihood of being diabetic, and (4) this score was used as an adjustment factor for all subsequent analyses. Accuracy of the propensity score was checked using receiver operating curve analysis. Calibration of the propensity score was checked with Hosmer-Lemeshow statistics.
Morbidity and mortality rates for each factor in the diabetic and nondiabetic groups were assessed using univariate and multivariable logistic regression analyses, respectively, obtaining crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs).
Sensitivity analysis for subgroups of isolated coronary, coronary plus valve, and valve operations was performed.
Statistical significance was settled at a p value <0.05; all tests were 2-sided; statistical analyses were performed with SPSS 13.0 (SPSS, Inc., Chicago, Illinois).
Results
The number of diabetic patients in our population was 1,480 (13.8%), with 9,229 nondiabetic patients. The 2 groups differed significantly in demographics, co-morbidities, and operative details ( Table 1 ). The diabetic group had a more severe risk profile due to older age, lower baseline hematocrit and ejection fraction, higher serum creatinine level, higher rate of many co-morbidities (long-term dialytic treatment, recent myocardial infarction, unstable angina, congestive HF, previous vascular and cardiac operations, chronic obstructive pulmonary disease, previous cerebrovascular accident), and a longer CPB duration. Patients in the nondiabetic group had a slightly lower rate of CABG plus valve operations, a lower rate of isolated CABG operations, and a higher rate of valve operations.
| Variable | Nondiabetics (n = 9,229) | Diabetics (n = 1,480) | p Value |
|---|---|---|---|
| Age (years) | 65.5 ± 11.7 | 67.6 ± 8.5 | 0.001 |
| Men | 6,406 (69%) | 1,011 (68%) | 0.394 |
| Weight (kg) | 73.1 ± 14.7 | 75.5 ± 14.4 | 0.001 |
| Hematocrit (%) | 39.4 ± 4.5 | 38.1 ± 4.7 | 0.001 |
| Serum creatinine (mg/dl) | 1.19 ± 0.8 | 1.35 ± 0.9 | 0.001 |
| Long-term dialytic treatment | 61 (0.7%) | 26 (1.8%) | 0.001 |
| Left ventricular ejection fraction | 0.52 ± 0.11 | 0.49 ± 0.12 | 0.001 |
| Recent myocardial infarction | 1,352 (14.6%) | 367 (24.8%) | 0.001 |
| Unstable angina pectoris | 560 (6.1%) | 110 (7.4%) | 0.044 |
| Congestive heart failure | 414 (4.5%) | 127 (8.6%) | 0.001 |
| Preoperative intra-aortic balloon pump | 50 (0.5%) | 15 (1.0%) | 0.030 |
| Active endocarditis | 60 (0.7%) | 8 (0.5%) | 0.622 |
| Previous vascular surgery | 364 (7.9%) | 107 (7.2%) | 0.001 |
| Previous cardiac surgery | 490 (5.3%) | 57 (3.9%) | 0.018 |
| Chronic obstructive pulmonary disease | 617 (6.7%) | 152 (10.3%) | 0.001 |
| Previous cerebrovascular accident | 402 (4.4%) | 110 (7.4%) | 0.001 |
| Isolated coronary artery bypass grafting | 4,545 (49.2%) | 1,012 (68.4%) | 0.001 |
| Coronary artery bypass grafting plus valve operation | 1,497 (16.2%) | 278 (18.7%) | 0.015 |
| Valve operation | 3,128 (33.9%) | 209 (14.1%) | 0.001 |
| Urgent operation | 369 (4.0%) | 72 (4.9%) | 0.119 |
| Cardiopulmonary bypass duration (minutes) | 75.6 ± 37 | 72.2 ± 34 | 0.001 |
| Cross-clamp time (minutes) | 50 ± 26 | 45.7 ± 23 | 0.001 |
Twelve factors ( Table 2 ) were independently associated with diabetes and were used for developing the propensity score.
| Factor With Diabetes | Regression Coefficient | p Value for Association |
|---|---|---|
| Age (years) | 0.009 | 0.002 |
| Weight (kg) | 0.013 | 0.001 |
| Hematocrit (%) | −0.053 | 0.001 |
| Serum creatinine (mg/dl) | 0.090 | 0.002 |
| Left ventricular ejection fraction | −0.012 | 0.001 |
| Recent myocardial infarction | 0.262 | 0.001 |
| Congestive heart failure | 0.518 | 0.001 |
| Previous vascular surgery | 0.350 | 0.004 |
| Chronic obstructive pulmonary disease | 0.282 | 0.005 |
| Previous cerebrovascular accident | 0.372 | 0.002 |
| Combined operation | 0.606 | 0.001 |
| Isolated coronary artery bypass grafting | 1.140 | 0.001 |
| Constant | −1.836 |
According to this score, the patient population was stratified for the propensity of belonging to the diabetic group, with a likelihood of 1.6% to 85.4%. Accuracy of the propensity score was confirmed by receiver operating curve analysis, which revealed an area under the curve of 0.72 (95% CI 0.706 to 0.734, p = 0.001) and the calibration was confirmed by Hosmer-Lemeshow statistics (chi-square 11.5, p = 0.175).
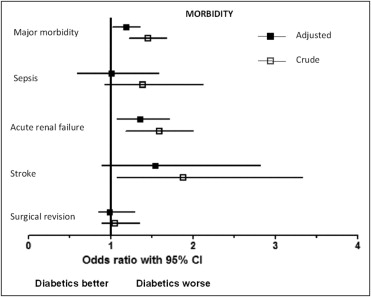
At univariate analysis, diabetic patients demonstrated a worse outcome, with longer mechanical ventilation time, longer intensive care unit and hospital stay, and higher rate of major morbidity, acute renal failure, stroke, and mortality ( Figures 1 and 2 , Table 3 ). Rates of myocardial infarction were 2% in nondiabetic patients and 1.6% in diabetic patients (p = 0.356). Conversely, diabetic patients showed a higher rate of acute respiratory insufficiency (3.7% vs 2.5% in nondiabetic patients, p = 0.005). When adjusted for propensity score, diabetic patients still demonstrated a longer hospital stay and a higher risk of major morbidity and acute renal failure but not a different mortality risk. The OR for major morbidity was 1.19 (95% CI 1.02 to 1.39, p = 0.024) and the OR for acute renal failure was 1.36 (95% CI 1.08 to 1.32, p = 0.01).