Percutaneous coronary intervention (PCI) to aorto-ostial (AO) lesions is technically demanding and associated with high revascularization rates. The aim of this study was to assess outcomes after bare metal stent (BMS) compared to drug-eluting stent (DES) implantation after PCI to AO lesions. A retrospective cohort analysis was conducted of all consecutive patients who underwent PCI to AO lesions at 2 centers. Angiographic and clinical outcomes in 230 patients with DES from September 2000 to December 2009 were compared to a historical control group of 116 patients with BMS. Comparison of the baseline demographics showed more diabetics (32% vs 16%, p = 0.001), lower ejection fractions (52.3 ± 9.7% vs 55.0 ± 11.5%, p = 0.022), longer stents (17.55 ± 7.76 vs 14.37 ± 5.60 mm, p <0.001), and smaller final stent minimum luminal diameters (3.43 ± 0.53 vs 3.66 ± 0.63 mm, p = 0.001) in the DES versus BMS group. Angiographic follow-up (DES 68%, BMS 66%) showed lower restenosis rates with DES (20% vs 47%, p <0.001). At clinical follow-up, target lesion revascularization rates were lowest with DES (12% vs 27%, p = 0.001). Cox regression analysis with propensity score adjustment for baseline differences suggested that DES were associated with a reduction in target lesion revascularization (hazard ratios 0.28, 95% confidence interval 0.15 to 0.52, p <0.001) and major adverse cardiac events (hazard ratio 0.50, 95% confidence interval 0.32 to 0.79, p = 0.003). There was a nonsignificantly higher incidence of Academic Research Consortium definite and probable stent thrombosis with DES (n = 9 [4%] vs n = 1 [1%], p = 0.131). In conclusion, despite differences in baseline characteristics favoring the BMS group, PCI with DES in AO lesions was associated with improved outcomes, with lower restenosis, revascularization, and major adverse cardiac event rates.
Various techniques have been tried in an attempt to improve angiographic and clinical outcomes in aorto-ostial (AO) lesions, such as the use of laser-based systems, directional atherectomy, cutting balloon predilatation, and implantation of polytetrafluoroethylene stents. The introduction of drug-eluting stents (DES) led to improved revascularization rates compared to bare-metal stent (BMS) implantation in AO lesions, but initial studies were relatively small and were performed before the availability of large DES with diameters >3.5 mm, leading to a possible discrepancy between the stent and reference vessel sizes. The aims of this study were to assess long-term angiographic and clinical outcomes after DES implantation in AO lesions in the contemporary percutaneous coronary intervention (PCI) era and to compare these results to a historical control group of BMS-treated lesions.
Methods
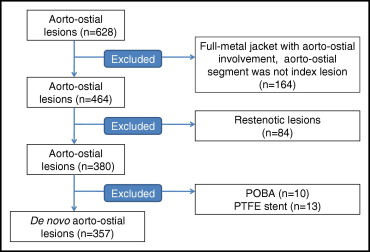
We analyzed data that had been prospectively collected after PCI at 2 institutions, San Raffaele Scientific Institute and EMO Centro Cuore Columbus Hospital (Milan, Italy). In total, 230 consecutive unselected patients with de novo AO disease treated exclusively with DES implantation from September 2002 to December 2009 were enrolled ( Figure 1 ) . Long-term outcomes after PCI to AO lesions with DES were compared to a historical control cohort of 116 consecutive unselected patients treated with BMS before the availability of DES. Exclusion criteria were PCI for ST-segment elevation myocardial infarction, PCI for restenosis, and PCI to an AO lesion as part of “full metal jacket” stent implantation.
An AO lesion was defined as a lesion involving the junction between the aorta and the orifice of the left main stem, right coronary artery, or a saphenous vein graft within 3 mm of the ostium of the vessel. Angiographic success was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow with <30% residual stenosis at the end of the procedure. Periprocedural myocardial infarction was defined as a 3-fold increase in creatine kinase-MB. Angiographic restenosis was defined as diameter stenosis ≥50% by quantitative coronary angiography within a previously stented segment or within 5 mm of the distal or proximal edge of the stent on follow-up angiography. The Mehran classification system was used to define the pattern of restenosis. Target lesion revascularization (TLR) was defined as the need for any repeat revascularization, either surgical or percutaneous, for a stenosis within the stent or within the 5-mm borders adjacent to the stent. Target vessel revascularization (TVR) was defined as the need for any repeat revascularization on a treated vessel. Major adverse cardiac events (MACE) were defined as a combination of all-cause mortality, nonfatal myocardial infarction, TLR, TVR, and need for coronary artery bypass grafting. Stent thrombosis was defined on the basis of the Academic Research Consortium definitions and cumulative stent thrombosis as a combination of all episodes of stent thrombosis during follow-up.
All patients were treated with aspirin and a loading dose of a thienopyridine before the procedure. During the procedure, all patients were then treated with intravenous heparin with an initial 100 U/kg bolus, followed by further heparin as required to achieve a target activated clotting time ≥250 seconds. Glycoprotein IIb/IIIa inhibitors were given at the operator’s discretion. Coronary intervention was then performed as usual with predilatation and stent implantation using standard techniques through the femoral artery, as previously described. Although the final interventional strategy was left to the discretion of the primary operator, most stents were implanted to protrude 1 mm into the aorta and were postdilated to high pressure with compliant or noncompliant balloon inflation. Postoperatively, all patients received aspirin unless there was a specific contraindication, and those patients receiving intracoronary stents received dual-antiplatelet therapy with aspirin and a thienopyridine as determined by contemporary guidelines. After the cessation of thienopyridine therapy, all patients continued to take aspirin indefinitely.
Cine angiograms were assessed using a validated edge detection system (CMS version 5.2; Medis Medical Imaging, Leiden, The Netherlands). All coronary angiograms were assessed by 2 experienced angiographers who were not involved in the stenting procedure and were blinded to the type of stent implanted. Angiographic analysis was performed using standard quantitative and qualitative methods, as previously described. Paired cine frames of 2 orthogonal frames in the end-systolic frames showing the stenosis in its most severe projection were selected. The minimum luminal diameter, reference vessel diameter, and percentage stenosis before and after coronary intervention and, when available, at follow-up were calculated. In addition, lesion length was analyzed before PCI. Angiographic restenosis was defined as >50% diameter stenosis by quantitative coronary angiography within a previously stented segment. Late luminal loss was calculated as the difference between minimum luminal diameter at the end of the procedure and the minimum luminal diameter on the initial angiogram at follow-up.
As is our regular clinical practice, follow-up was obtained at regular intervals with clinic visits or telephone interviews at 3, 6, and 12 months in the first year and at intervals of 6 months thereafter. Additional data were obtained from primary care physicians, referring cardiologists, or relatives as necessary. All repeat interventions and complications were prospectively entered into a dedicated database. All adverse events were clarified by reviewing the medical notes or contacting the patient’s physician. Clinical follow-up was obtained in 94% (n = 326). Our regular clinical practice is to follow up all high-risk PCI procedures with angiographic surveillance when possible at 6 to 9 months after the index procedure. Angiographic follow-up was obtained in 67% of lesions (n = 227). Functional test results were negative in those patients in whom angiographic follow-up was not performed.
Adverse procedural and in-hospital events were defined as the need for cardiopulmonary resuscitation, myocardial infarction, acute stent thrombosis, necessity for urgent coronary artery bypass grafting, and death. The long-term primary end points were defined as death from any cause, myocardial infarction, TVR, TLR, need for coronary artery bypass grafting, and MACE at any time during the in-hospital stay or at follow-up. We defined the secondary end points as the incidence of restenosis, and stent thrombosis, defined as probable and definite.
Statistical analysis was performed using SPSS version 16.0 (SPSS, Inc., Chicago, Illinois). Continuous variables are expressed as mean ± SD or as medians with interquartile ranges as appropriate. Categorical variables are expressed as counts and percentage. Continuous variables were compared using Student’s t tests. Categorical variables were compared using chi-square statistics. A p value <0.05 was considered statistically significant, and all reported p values are 2 sided.
To account for potential differences between the 2 groups, a propensity analysis was performed on a patient-based setting for MACEs and a lesion-based setting for TLR. The propensity to be treated with DES was estimated using a nonparsimonious multivariate logistic regression model. The individual propensity score was then incorporated into Cox proportional-hazards regression models as a covariate as well as a treatment group (i.e., DES vs BMS) to calculate the adjusted hazard ratios (HRs). Variables included in the propensity model were age, gender, the ejection fraction, hypertension, hypercholesterolemia, diabetes, unstable angina, current smoking, previous PCI, family history of coronary artery disease, left main stem treatment, right coronary artery treatment, total stent length, number of stents per lesion, rotablation, postdilatation balloon diameter, direct stenting, and intravascular ultrasound guidance. In the lesion-based analysis, because observations recorded in the same patient cannot be considered independent, the sandwich estimator of the variance-covariance matrix was used to take into account clustered data (more lesions within the same subject). The discrimination and calibration ability of the propensity score model was assessed by means of the C-statistic and the Hosmer-Lemeshow statistic. The results of the Cox proportional-hazards analyses are reported as unadjusted and adjusted HRs with associated 95% confidence intervals (CIs) and p values.
Results
From a total of 628 AO lesions recorded in our database, we excluded 271 lesions ( Figure 1 ). This resulted in a final group of 357 de novo AO lesions in 346 patients, with 239 lesions (230 patients) in the DES group and 118 lesions (116 patients) in the BMS group. The baseline clinical demographics of these patients are presented in Table 1 . There were some baseline differences in clinical demographics between the 2 groups, with a higher incidence of previous PCI, hypercholesterolemia, and diabetes mellitus in the DES group.
| Variable | BMS (n = 116) | DES (n = 230) | p Value |
|---|---|---|---|
| Age (years) | 68.6 ± 10.6 | 65.7 ± 10.88 | 0.019 † |
| Men | 79 (68%) | 174 (76%) | 0.135 |
| Ejection fraction (%) | 55.0 ± 11.5 | 52.3 ± 9.7 | 0.022 † |
| Previous myocardial infarction | 41 (35%) | 101 (44%) | 0.126 |
| Previous PCI | 28 (24%) | 128 (56%) | <0.001 † |
| Previous coronary artery bypass grafting | 41 (35%) | 86 (66%) | 0.709 |
| Hypertension ⁎ | 70 (60%) | 162 (70%) | 0.059 |
| Hypercholesterolemia ⁎ | 68 (59%) | 167 (73%) | 0.009 † |
| Current smoker | 13 (11%) | 13 (6%) | 0.064 |
| Diabetes mellitus | 18 (16%) | 73 (32%) | 0.001 † |
| Unstable angina pectoris (CCS class IV) | 44 (38%) | 51 (22%) | 0.002 † |
| Stable angina pectoris (CCS class I–III) | 57 (49%) | 99 (43%) | 0.282 |
| Silent myocardial ischemia (CCS class 0) | 14 (12%) | 59 (26%) | 0.003 † |
| Multivessel coronary disease | 97 (84%) | 197 (86%) | 0.401 |
| Number coronary arteries diseased | |||
| 1 | 19 (16%) | 33 (14%) | 0.618 |
| 2 | 35 (30%) | 67 (29%) | 0.841 |
| 3 | 62 (53%) | 130 (57%) | 0.587 |
⁎ Defined as being treated before coronary angiography.
The baseline lesion characteristics are listed in Table 2 . In the BMS and DES groups, most lesions were located within the right coronary artery. There were no significant differences in preintervention lesion characteristics between the groups as assessed by quantitative coronary angiography.
| Variable | BMS (n = 118) | DES (n = 239) | p Value |
|---|---|---|---|
| AO lesion | |||
| Left main coronary artery | 40 (34%) | 91 (38%) | 0.441 |
| Right coronary artery | 67 (57%) | 108 (45%) | 0.039 ⁎ |
| Saphenous vein graft | 11 (9%) | 40 (17%) | 0.060 |
| Lesion characteristics | |||
| Type B1 | 32 (27%) | 85 (36%) | 0.110 |
| Type B2 | 64 (55%) | 110 (46%) | 0.144 |
| Type C | 22 (19%) | 44 (18%) | 0.957 |
| Total occlusion | 1 (1%) | 4 (2%) | 0.532 |
| Before intervention | |||
| Reference vessel diameter (mm) | 3.49 ± 0.83 | 3.32 ± 0.84 | 0.097 |
| Minimum luminal diameter (mm) | 1.36 ± 0.68 | 1.31 ± 0.61 | 0.582 |
| Stenosis (%) | 62.59 ± 16.35 | 60.96 ± 16.99 | 0.434 |
| Lesion length (mm) | 6.63 ± 4.68 | 7.87 ± 5.13 | 0.057 |
The baseline procedural characteristics are listed in Table 3 . Multivessel PCI was performed in most patients in the 2 groups. The total stent length was longer in the DES group than in the BMS group (17.55 ± 7.76 vs 14.37 ± 5.60 mm, p <0.001). The final stent minimum luminal diameter was significantly smaller in the DES group (3.43 ± 0.53 vs 3.66 ± 0.63 mm, p = 0.001). Balloon predilatation and intravascular ultrasound were performed less frequently in the DES group.
| Variable | BMS (n = 118) | DES (n = 239) | p Value |
|---|---|---|---|
| Glycoprotein IIb/IIIa inhibitors | 12 (10%) | 16 (7%) | 0.252 |
| Multivessel stenting | 98 (83%) | 200 (84%) | 0.880 |
| Number of stents per lesion | 1.02 ± 0.13 | 1.05 ± 0.23 | 0.127 |
| DES implanted | 246 | ||
| Sirolimus-eluting stent | — | 114 (46%) | |
| Paclitaxel-eluting stent | — | 89 (36%) | |
| Everolimus-eluting stent | — | 24 (10%) | |
| Zotarolimus-eluting stent | — | 16 (7%) | |
| Biolimus-eluting stent | — | 3 (1%) | |
| Total stent length (mm) | 14.37 ± 5.60 | 17.55 ± 7.76 | <0.001 ⁎ |
| Final stent minimal luminal diameter (mm) | 3.66 ± 0.63 | 3.43 ± 0.53 | 0.001 ⁎ |
| Direct stenting | 23 (20%) | 74 (31%) | 0.022 ⁎ |
| Balloon predilatation | 88 (75%) | 116 (49%) | <0.001 ⁎ |
| Cutting balloon predilatation | 5 (4%) | 22 (9%) | 0.095 |
| Directional coronary atherectomy | 10 (9%) | 0 | <0.001 ⁎ |
| Intravascular ultrasound guidance | 37 (31%) | 41 (17%) | 0.002 ⁎ |
| Rotablation | 16 (14%) | 1 (1%) | <0.001 ⁎ |
| Noncompliant balloon postdilatation | 65 (55%) | 99 (41%) | 0.015 ⁎ |
| Postdilatation balloon diameter (mm) | 3.43 ± 1.37 | 2.74 ± 1.72 | <0.001 ⁎ |
| Postdilatation balloon length (mm) | 14.55 ± 7.48 | 11.29 ± 8.77 | <0.001 ⁎ |
| Maximum balloon inflation (atm) | 15.31 ± 7.25 | 15.72 ± 10.67 | 0.666 |
Table 4 lists the angiographic outcomes at follow-up. At follow-up, the minimum luminal diameter was significantly larger in the DES group than in the BMS group (2.97 ± 0.95 vs 2.21 ± 1.15 mm, p <0.001). In addition, the incidence of binary restenosis was 20% (32 of 157) in the DES group compared to 47% (32 of 70) in the BMS group (p <0.001).
| Angiographic Outcome | BMS (n = 107) | DES (n = 231) | p Value |
|---|---|---|---|
| Angiographic follow-up obtained | 70 (66%) | 157 (68%) | 0.726 |
| Postintervention | |||
| Reference vessel diameter (mm) | 3.85 ± 0.68 | 3.79 ± 0.57 | 0.449 |
| Minimum luminal diameter (mm) | 3.66 ± 0.63 | 3.43 ± 0.53 | 0.001 ⁎ |
| Stenosis (%) | 3.99 ± 11.01 | 9.23 ± 7.91 | <0.001 ⁎ |
| Follow-up | |||
| Reference vessel diameter (mm) | 3.50 ± 0.65 | 3.64 ± 0.57 | 0.196 |
| Minimum luminal diameter (mm) | 2.21 ± 1.15 | 2.97 ± 0.95 | <0.001 ⁎ |
| Stenosis (%) | 38.83 ± 28.62 | 18.03 ± 21.24 | <0.001 ⁎ |
| Lesion length (mm) | 5.42 ± 4.52 | 4.94 ± 3.59 | 0.519 |
| Late luminal loss (mm) | 1.54 ± 1.21 | 0.42 ± 0.84 | <0.001 ⁎ |
| Restenosis | 32 (47%) | 32 (20%) | <0.001 ⁎ |
| Restenosis type | |||
| Focal | 22 (69%) | 19 (59%) | 0.434 |
| Diffuse | 7 (22%) | 9 (28%) | 0.564 |
| Occlusive | 3 (9%) | 4 (13%) | 0.689 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


