Coronary heart disease is a major risk factor for left ventricular (LV) systolic dysfunction. However, limited data are available regarding long-term benefits of percutaneous coronary intervention (PCI) in the era of drug-eluting stent or coronary artery bypass grafting (CABG) in patients with LV systolic dysfunction with severe coronary artery disease. We identified 3,584 patients with 3-vessel and/or left main disease of 15,939 patients undergoing first myocardial revascularization enrolled in the CREDO-Kyoto PCI/CABG Registry Cohort-2. Of them, 2,676 patients had preserved LV systolic function, defined as an LV ejection fraction (LVEF) of >50% and 908 had impaired LV systolic function (LVEF ≤50%). In patients with preserved LV function, 5-year outcomes were not different between PCI and CABG regarding propensity score–adjusted risk of all-cause and cardiac deaths. In contrast, in patients with impaired LV systolic function, the risks of all-cause and cardiac deaths after PCI were significantly greater than those after CABG (hazard ratio 1.49, 95% confidence interval 1.04 to 2.14, p = 0.03 and hazard ratio 2.39, 95% confidence interval 1.43 to 3.98, p <0.01). In both patients with moderate (35% < LVEF ≤ 50%) and severe (LVEF ≤35%) LV systolic dysfunction, the risk of cardiac death after PCI was significantly greater than that after CABG (hazard ratio 2.25, 95% confidence interval 1.15 to 4.40, p = 0.02 and hazard ratio 4.42, 95% confidence interval 1.48 to 13.24, p = 0.01). Similarly, the risk of all-cause death tended to be greater after PCI than after CABG in both patients with moderate and severe LV systolic dysfunction without significant interaction (hazard ratio 1.57, 95% confidence interval 0.96 to 2.56, p = 0.07 and hazard ratio 1.42, 95% confidence interval 0.71 to 2.82, p = 0.32; interaction p = 0.91). CABG was associated with better 5-year survival outcomes than PCI in patients with impaired LV systolic function (LVEF ≤50%) with complex coronary disease in the era of drug-eluting stents. In both patients with moderate (35% < LVEF ≤ 50%) and severe (LVEF ≤35%) LV systolic dysfunction, CABG tended to have better survival outcomes than PCI.
Highlights
- •
Outcomes after percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were compared in patients with ejection fractions (EFs) ≤50% versus >50%.
- •
Similar outcomes were found between PCI and CABG in patients with EF >50%.
- •
CABG had a better survival rate than PCI in patients with EF ≤50%.
- •
Further study is warranted in patients with severe LV systolic dysfunction (EF ≤35%).
Coronary artery disease is the most common cause of left ventricular (LV) systolic dysfunction and has increased with the growing incidence of associated mortality. Several studies have shown improved outcomes after coronary artery bypass grafting (CABG) in patients with low LV ejection fraction (LVEF). However, limited reports are available regarding the benefits of percutaneous coronary intervention (PCI) in patients with LV systolic dysfunction. Furthermore, in the drug-eluting stent (DES) era, few reports have compared the clinical outcomes of CABG and DES implantation in patients with LV systolic dysfunction, particularly in more complex coronary lesions such as 3-vessel or left main disease. In the present study, therefore, we mainly compared the 5-year outcomes between PCI and CABG in patients with preserved LV systolic function (LVEF >50%) or impaired LV systolic function (LVEF ≤50%) accompanying with 3-vessel and/or left main disease using a large observational database in Japan. As a subanalysis, we compared the outcomes between PCI and CABG in patients with moderate (35% < LVEF ≤ 50%) and severe (LVEF ≤35%) LV systolic dysfunction.
Methods
The Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) PCI/CABG Registry Cohort-2 is a physician-initiated, noncompany-sponsored, multicenter registry that enrolled consecutive patients undergoing first coronary revascularization in the 26 centers in Japan from January 2005 through December 2007. The relevant ethics committees in all 26 participating centers (see Supplementary Data A ) approved the research protocol. Because of retrospective enrollment, written informed consent from the patients was waived. However, patients who refused participation in the study when contacted for follow-up were excluded.
The study design and patient enrollment in the registry have been described in detail previously. Of the 15,939 patients enrolled in the registry, patients who refused study participation, who had concomitant noncoronary surgery, who had acute myocardial infarction (MI), and who had single- or double-vessel disease were excluded. For the comparison of PCI with CABG, we selected 3,982 patients with 3-vessel and/or left main disease. Excluding 298 patients (10%) without LVEF data, the present study population consisted of 3,584 patients with 3-vessel and/or left main disease with known LVEF. There were 2,676 patients with preserved LV systolic function, defined as an LVEF of >50%, and 908 patients with impaired LV systolic function (LVEF ≤50%).
LVEF was measured by echocardiography or LV cine angiography. M-mode and/or 2-dimensional echocardiography was performed by experienced operators in each institution. M-mode LVEF was calculated using the Teichholz correction. Two-dimensional echocardiographic LVEF was also evaluated by the Simpson biplane method of discs with manual planimetry of the endocardial border in end-diastolic (largest) and end-systolic (smallest) frames. LV cine angiography was obtained in either a single-plane right anterior oblique view or biplane right and left anterior oblique views. LVEF was calculated using the built-in programs.
Demographic, angiographic, and procedural data were collected from hospital charts according to the prespecified definitions by experienced research coordinators in an independent research organization (Research Institute for Production Development, Kyoto, Japan; see Supplementary Data B ). Definitions for clinical characteristics are presented in the Supplementary Data C .
The Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score was calculated using the SYNTAX score calculator (available at: http://www.syntaxscore.com ) by a dedicated SYNTAX score committee (see Supplementary Data D ) in a blinded fashion to the clinical data. Intraobserver and interobserver variabilities of the SYNTAX score calculation in our group were previously reported. Cut-off values for SYNTAX score tertiles (low <23, intermediate 23 to 33, and high ≥33) were defined according to the analysis in the SYNTAX trial.
Collection of follow-up information was conducted mainly through review of inpatient and outpatient hospital charts by clinical research coordinators in the independent research organization. Additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by sending mail with questions on vital status and additional hospitalizations. Death, MI, stent thrombosis, and stroke were adjudicated by the clinical event committee (see Supplementary Data E ).
The primary outcome measure for the present analysis was death from any cause. Other prespecified end points included cardiac death, sudden death, stroke, MI, and any coronary revascularization. Death was regarded as cardiac in origin unless obvious noncardiac causes could be identified. Any death during the index hospitalization for coronary revascularization was regarded as cardiac death. Sudden death was defined as an unexpected death in previously stable patients. MI was defined according to the definition in the Arterial Revascularization Therapy Study. Stroke during follow-up was defined as an ischemic or a hemorrhagic stroke requiring hospitalization with symptoms lasting >24 hours. Scheduled staged coronary revascularization procedures performed within 3 months of the initial procedure were not regarded as follow-up events but were included in the index procedure.
All continuous variables are expressed as the mean ± SD. Differences in baseline characteristics between the 2 groups were examined by the unpaired t test and Fisher’s exact test. Cumulative incidence was estimated by the Kaplan-Meier method, and differences were assessed using the log-rank test. Propensity scores, which were the probabilities that a patient would undergo PCI, were estimated with multivariate logistic regression analyses including age, gender, body mass index, hypertension, dyslipidemia, diabetes mellitus, current smoker, heart failure, mitral regurgitation grade 3 or 4, previous MI, previous stroke, peripheral arterial disease, atrial fibrillation, chronic kidney disease, hemodialysis, anemia, platelet count, chronic obstructive lung disease, liver cirrhosis, malignancy, emergency procedure, number of diseased vessels, left main disease, target chronic total occlusion, target proximal left anterior descending coronary artery, and SYNTAX score as the covariates. These variables were consistent with previous reports from the present registry. Continuous variables, except age, were dichotomized using clinically meaningful reference values or median values. We incorporated the 26 participating centers in the propensity score estimation as the stratification variable. The hazard ratios of PCI compared with those of CABG were estimated by the stratified Cox proportional hazard models; the models included PCI or CABG as the covariate and were stratified by the quartiles of propensity score and institute to adjust for confounding. Effects of PCI compared with those of CABG for individual end points are expressed as hazard ratios with 95% confidence intervals. All reported p values were 2-sided, and p values <0.05 were regarded as statistically significant.
All analyses were conducted by a statistician using SAS software, version 9.3 (SAS Institute Inc, Cary, NC), and S-Plus, version 7.0 (Insightful Corp). The investigators had full access to the data and take responsibility for its integrity. All the investigators have read and agreed to the manuscript as written.
Results
Baseline clinical characteristics comparing the PCI with CABG groups in patients with preserved and impaired LV systolic functions are listed in Tables 1 and 2 . LVEF was not different between PCI and CABG in both patients with preserved and impaired LV systolic functions. The SYNTAX score was higher in the CABG group in both patients with preserved and impaired LV systolic functions. The mean follow-up period was 4.74 years (median 5.12 years). Completeness of the follow-up for 3, 4, and 5 years was 95.8%, 94.4%, and 70.9%, respectively.
| Variable | PCI n = 1432 | CABG n = 1244 | p Value |
|---|---|---|---|
| Age (years) | 69.7 ± 9.7 | 68.5 ± 8.7 | <0.001 |
| >75 | 495 (35%) | 338 (27%) | <0.001 |
| Men | 998 (70%) | 914 (73%) | 0.03 |
| Ejection fraction (%) | 65.0 ± 7.8 | 65.0 ± 8.5 | 0.89 |
| Previous myocardial infarction | 152 (11%) | 178 (14%) | 0.004 |
| Heart failure | 137 (10%) | 135 (11%) | 0.27 |
| Atrial fibrillation | 94 (7%) | 229 (18%) | <0.001 |
| Mitral regurgitation grade 3/4 | 52 (4%) | 24 (2%) | 0.08 |
| Body mass index (kg/m 2 ) | 24.0 ± 3.5 | 23.6 ± 3.1 | 0.02 |
| >25 | 498 (35%) | 390 (31%) | 0.06 |
| Hypertension | 1236 (86%) | 1057 (85%) | 0.32 |
| Diabetes mellitus | 660 (46%) | 627 (50%) | 0.03 |
| On insulin therapy | 170 (12%) | 197 (16%) | 0.003 |
| Current smoker | 342 (24%) | 280 (23%) | 0.40 |
| Previous stroke | 207 (14%) | 159 (13%) | 0.21 |
| Peripheral artery disease | 170 (12%) | 151 (12%) | 0.83 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 62.3 ± 21.8 | 58.1 ± 23.7 | <0.001 |
| Hemodialysis | 50 (3%) | 67 (5%) | 0.02 |
| Anemia (hemoglobin <11.0 g/dl) | 182 (13%) | 220 (18%) | <0.001 |
| Platelet count <100 × 10 9 /L | 22 (2%) | 28 (2%) | 0.17 |
| Chronic obstructive pulmonary disease | 40 (3%) | 26 (2%) | 0.24 |
| Liver cirrhosis | 47 (3%) | 38 (3%) | 0.74 |
| Malignancy | 178 (12%) | 139 (11%) | 0.32 |
| Procedural characteristics | |||
| Number of target coronary narrowings or anastomoses | 2.0 ± 1.0 | 3.3 ± 1.1 | <0.001 |
| Emergency procedure | 42 (3%) | 40 (3%) | 0.67 |
| Percutaneous coronary intervention | |||
| Stent use | 1370 (96%) | — | n/a |
| Drug-eluting stent use | 1039 (73%) | — | n/a |
| Coronary artery bypass grafting | |||
| Left internal thoracic artery use | — | 1202 (97%) | n/a |
| Bilateral internal thoracic artery use | — | 381 (31%) | n/a |
| Off-pump coronary artery bypass grafting | — | 832 (67%) | n/a |
| Number of coronary arteries narrowed: | |||
| 3 (Without left main disease) | 1220 (85%) | 783 (63%) | <0.001 |
| Left main | 212 (15%) | 461 (37%) | <0.001 |
| Proximal left anterior descending artery | 879 (61%) | 1073 (86%) | <0.001 |
| Chronic total occlusion | 251 (18%) | 460 (37%) | <0.001 |
| SYNTAX score | 23.3 ± 9.3 | 29.6 ± 11.6 | <0.001 |
| Low (<23) | 708 (50%) | 318 (29%) | <0.001 |
| Intermediate (23–32) | 485 (35%) | 401 (36%) | |
| High (≥33) | 211 (15%) | 384 (35%) |
| Variable | PCI n = 464 | CABG n = 444 | p Value |
|---|---|---|---|
| Age (years) | 70.1 ± 10.4 | 67.8 ± 9.7 | <0.001 |
| >75 | 186 (40%) | 128 (29%) | <0.001 |
| Men | 354 (76%) | 347 (78%) | 0.50 |
| Ejection fraction (%) | 38.7 ± 8.7 | 39.2 ± 8.3 | 0.40 |
| Previous myocardial infarction | 202 (44%) | 201 (45%) | 0.60 |
| Heart failure | 251 (54%) | 224 (50%) | 0.27 |
| Atrial fibrillation | 57 (12%) | 77 (17%) | 0.02 |
| Mitral regurgitation grade 3/4 | 66 (14%) | 27 (6%) | <0.001 |
| Body mass index (kg/m 2 ) | 23.4 ± 3.5 | 22.7 ± 3.3 | <0.001 |
| >25 | 141 (30%) | 100 (23%) | 0.007 |
| Hypertension | 415 (89%) | 378 (85%) | 0.05 |
| Diabetes mellitus | 264 (57%) | 249 (56%) | 0.80 |
| On insulin therapy | 75 (16%) | 94 (21%) | 0.04 |
| Current smoker | 122 (26%) | 134 (30%) | 0.19 |
| Previous stroke | 84 (18%) | 71 (16%) | 0.40 |
| Peripheral artery disease | 60 (13%) | 73 (16%) | 0.13 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 52.6 ± 25.3 | 51.2 ± 24.6 | 0.40 |
| Hemodialysis | 51 (11%) | 43 (10%) | 0.52 |
| Anemia (hemoglobin <11.0 g/dl) | 104 (22%) | 98 (22%) | 0.90 |
| Platelet count <100 × 10 9 /L | 7 (2%) | 10 (2%) | 0.41 |
| Chronic obstructive pulmonary disease | 17 (4%) | 12 (3%) | 0.41 |
| Liver cirrhosis | 12 (3%) | 12 (3%) | 0.91 |
| Malignancy | 38 (8%) | 39 (9%) | 0.75 |
| Procedural characteristics | |||
| Number of target coronary narrowings or anastomoses | 2.1 ± 1.0 | 3.4 ± 1.0 | <0.001 |
| Emergency procedure | 12 (3%) | 17 (4%) | 0.29 |
| Percutaneous coronary intervention | |||
| Stent use | 420 (91%) | — | n/a |
| Drug-eluting stent use | 347 (75%) | — | n/a |
| Coronary artery bypass grafting | |||
| Left internal thoracic artery use | — | 430 (97%) | n/a |
| Bilateral internal thoracic artery use | — | 116 (26%) | n/a |
| Off-pump coronary artery bypass grafting | — | 236 (53%) | n/a |
| Number of coronary artery narrowed: | |||
| 3 (Without left main disease) | 389 (84%) | 318 (72%) | <0.001 |
| Left main | 75 (16%) | 126 (28%) | <0.001 |
| Proximal left anterior descending artery | 298 (64%) | 403 (91%) | <0.001 |
| Chronic total occlusion | 147 (32%) | 261 (59%) | <0.001 |
| SYNTAX score | 26.6 ± 9.8 | 32.5 ± 10.7 | <0.001 |
| Low (<23) | 165 (36%) | 80 (20%) | <0.001 |
| Intermediate (23–32) | 177 (39%) | 133 (33%) | |
| High (≥33) | 111 (25%) | 187 (47%) |
Kaplan-Meier analysis showed that cumulative incidence of all-cause death at 5 years was not different between PCI and CABG in patients with preserved LV systolic function ( Figure 1 ). However, in patients with impaired LV systolic function, the cumulative incidence of all-cause death after PCI was significantly greater than that after CABG. Similarly, cumulative incidences of cardiac death and readmission for heat failure were not different between PCI and CABG in patients with preserved LV systolic function but were significantly greater after PCI in patients with impaired LV systolic function ( Figure 1 ).
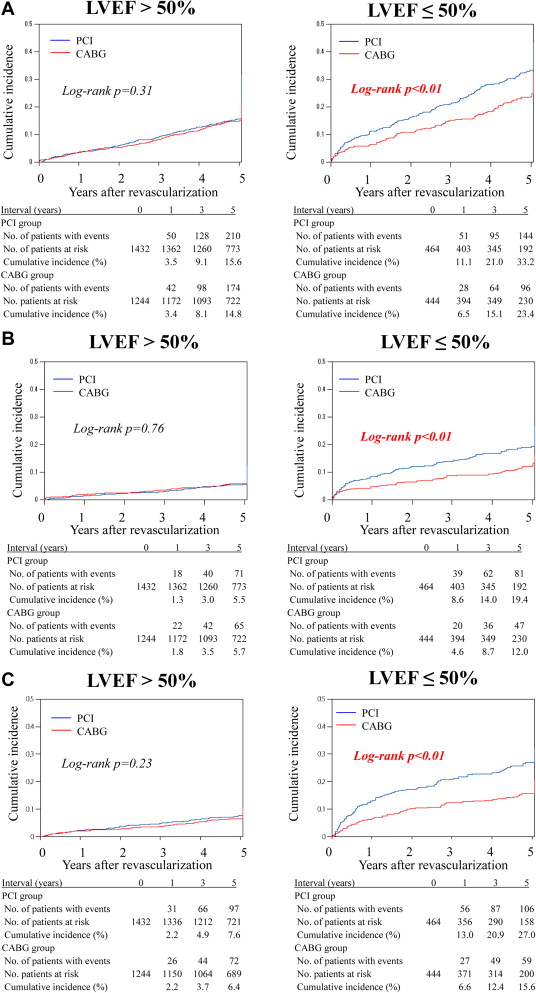
In patients with preserved LV systolic function, propensity score–adjusted risk of all-cause death, cardiac death, sudden death, readmission for heart failure, and stroke was not different between PCI and CABG ( Table 3 ). The risk of MI and repeat revascularization after PCI was greater than that after CABG. In contrast, in patients with impaired LV systolic function, all-cause mortality after PCI was significantly greater than that after CABG. Similarly, the risk of cardiac death and sudden death after PCI was significantly higher after PCI than after CABG. The risk of stroke and MI was not different between the groups.
| Variable | Number of Patients (Event/Total) | Comparison of PCI vs. CABG | |||||
|---|---|---|---|---|---|---|---|
| PCI | CABG | Hazard Ratio ∗ | 95% Confidence Interval | p | Interaction p | ||
| Death | |||||||
| LVEF >50% | 24 (17%) | 197 (16%) | 0.98 | 0.76 | 1.28 | 0.91 | 0.12 |
| LVEF ≤50% | 155 (33%) | 113 (25%) | 1.49 | 1.04 | 2.14 | 0.03 | |
| Cardiac death | |||||||
| LVEF >50% | 86 (6%) | 72 (6%) | 0.85 | 0.55 | 1.32 | 0.47 | 0.06 |
| LVEF ≤50% | 87 (19%) | 53 (12%) | 2.39 | 1.43 | 3.98 | <0.01 | |
| Sudden death | |||||||
| LVEF >50% | 37 (3%) | 22 (2%) | 1.77 | 0.87 | 3.61 | 0.12 | 0.61 |
| LVEF ≤50% | 28 (6%) | 15 (3%) | 2.45 | 1.01 | 5.95 | 0.05 | |
| Readmission for heart failure | |||||||
| LVEF >50% | 108 (8%) | 80 (6%) | 1.39 | 0.92 | 2.09 | 0.11 | 0.24 |
| LVEF ≤50% | 113 (24%) | 65 (15%) | 2.22 | 1.42 | 3.46 | <0.01 | |
| Stroke | |||||||
| LVEF >50% | 113 (8%) | 102 (8%) | 0.78 | 0.54 | 1.14 | 0.20 | 0.23 |
| LVEF ≤50% | 37 (8%) | 46 (10%) | 0.75 | 0.41 | 1.38 | 0.36 | |
| Myocardial infarction | |||||||
| LVEF >50% | 94 (7%) | 30 (2%) | 2.31 | 1.34 | 3.97 | <0.01 | 0.32 |
| LVEF ≤50% | 36 (8%) | 19 (4%) | 2.20 | 0.96 | 5.04 | 0.06 | |
| Any revascularization | |||||||
| LVEF >50% | 663 (46%) | 182 (15%) | 3.69 | 2.96 | 4.60 | <0.01 | 0.07 |
| LVEF ≤50% | 189 (41%) | 45 (10%) | 4.42 | 2.87 | 6.81 | <0.01 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


