Previous reports have shown the advantage of paclitaxel compared to limus-derivative drugs for the treatment of diabetics. A total of 109 diabetics (115 lesions) treated with everolimus-eluting stents (EESs, n = 58) or paclitaxel-eluting stents (PESs, n = 55) undergoing 8 to 9 months of follow-up 3-dimensional intravascular ultrasound examinations were enrolled. In addition to the standard intravascular ultrasound parameters, the percentage of neointimal volume (neointimal volume/stent volume) and maximum percentage of cross-sectional narrowing (neointimal area/stent area) was calculated. EESs showed a lower percentage of neointimal volume (7.2 ± 7.1% vs 11.7 ± 11.0%; p = 0.01) and maximum percentage of cross-sectional narrowing (22.5 ± 16.3% vs 29.4 ± 19.2%; p = 0.04) than PESs. One case of severe narrowing (lesions with maximum percentage of cross-sectional narrowing >60%) in the EES group developed and 6 cases in the PESs group (p = 0.05). The EESs showed no serial changes for vessel or peri-stent plaque during the follow-up period, and PESs showed significant increases in vessel and peri-stent plaque. PESs showed significantly greater peri-stent plaque increase, with a tendency toward greater vessel enlargement than EESs. Late acquired incomplete stent apposition was observed in 2 PES cases. The major adverse cardiac event rate was comparable ≤2 years. In conclusion, EESs showed greater neointimal suppression without significant vessel expansion than PESs in diabetic patients. In this small cohort, no significant differences were observed in the major adverse cardiac event rate ≤2 years.
Diabetes mellitus (DM) is still associated with an increased risk of restenosis and unfavorable clinical outcomes in the drug-eluting stent era. In the SPIRIT III trial, everolimus-eluting stents (EESs, XIENCE V, Abbott Vascular, Santa Clara, California) has shown a significant reduction in the 8-month angiographic late loss and 2-year major adverse cardiac events (MACE) rate compared to paclitaxel-eluting stents (PESs, TAXUS, Boston Scientific, Boston, Massachusetts). However, a significant interaction favoring PESs was observed between DM and stent type on MACE in the subgroup analysis of this trial. In addition, there is an argument favoring paclitaxel over limus-derivative drugs for the treatment of DM because of the theoretical hypothesis that the mammalian target of rapamycin (mTOR) might be degraded in insulin-resistant states. Therefore, the aim of the present study was to compare the arterial response to EESs versus PESs in patients with DM using 3-dimensional intravascular ultrasound (IVUS) evaluation with 2 years of clinical follow-up.
Methods
The original study data were pooled at the patient and lesion levels from 3 drug-eluting stent randomized trials: ZoMaxx I, ZoMaxx II, SPIRIT III, and one nonrandomized study, the SPIRIT III Japan registry. All studies were sponsored and monitored by the Abbot Vascular (Abbott Laboratories, Abbott Park, Illinois). The study design and 8- or 9-month results have been previously reported for those trials. In brief, the ZoMaxx I and II trial were prospective, multicenter, randomized trials in which patients were randomized in a 1:1 ratio to receive zotarolimus-eluting stents (ZoMaxx, Abbott Vascular) or the PESs. The ZoMaxx I trial included patients with a single de novo lesion 10 to 30 mm long with a diameter of 2.5 to 3.5 mm. The ZoMaxx II trial included patients with a single de novo lesion 10 to 28 mm long with a diameter of 2.5 to 3.75 mm. The SPIRIT III trial was a prospective, multicenter, randomized trial in which patients with 1 or 2 de novo lesions ≤28 mm long in a native vessel with a diameter of 2.5 to 3.75 mm were randomized in a 2:1 ratio to receive the EES or PES. The SPIRIT III Japan registry was a prospective, multicenter, nonrandomized arm to evaluate the safety and efficacy of EESs in the 88 consecutive Japanese patients with 1 or 2 de novo lesions ≤28 mm long in a native vessel with a diameter of 2.5 to 4.25 mm. The major exclusion criteria of these trials included acute myocardial infarction, stroke, impaired left ventricular function (ejection fraction <30%), ostial or left main lesions, and previously treated lesions. From these studies, the results of standard angiographic and IVUS analysis and clinical events for ≤2 years from patients meeting the following criteria were pooled: (1) patients with DM and stable or unstable angina who had undergone successful EES or PES implantation; (2) patients who were enrolled into a prespecified IVUS substudy. DM was defined as a history of DM requiring current treatment with insulin, oral agents, and/or dietary therapy. Patients treated with ZoMaxx were excluded. The institutional review board approved the protocol, and each patient provided written informed consent.
A follow-up IVUS examination was prospectively scheduled for all patients involved in the present study, regardless of symptoms, as a part of the clinical research protocols. The IVUS procedure was performed in a standard fashion using automated motorized 0.5 mm/s pullback with commercially available imaging systems (40-MHz IVUS catheter, Boston Scientific, Natick, Massachusetts, or 20-MHz IVUS catheter, Volcano, Rancho Cordova, California). All standard IVUS analyses were performed by the same independent core laboratory for all trials (Center for Cardiovascular Technology, Stanford University, Stanford, California) that was unaware of the treatment arm. Volumetric measurements were performed using planimetry software (EchoPlaque, Indec Systems, Santa Clara, California), as previously described. The volume index was calculated as the volume data divided by the length. To evaluate neointimal proliferation, the following 2 parameters were analyzed: (1) percentage neointimal volume (percentage of neointimal volume = neointimal volume/stent volume × 100) to assess the overall degree of neointimal proliferation; and (2) the maximum percentage of cross-sectional narrowing (maximum percentage of cross-sectional narrowing = neointimal area/stent area × 100) within each stented segment to assess the most severe effect of neointima on luminal encroachment. The lesions with maximum percentage of cross-sectional narrowing >60% were considered lesions with severe narrowing. For the assessment of edge restenosis, an additional subsegment analysis was conducted by dividing the stented segment into 3 subsegments (1) proximal in-stent (within 3 mm from the proximal stent edge); (2) stent body; and (3) distal in-stent (within 3 mm from the distal stent edge). Next, the neointimal volume index was compared between the EESs and PESs for each subsegment. Incomplete stent apposition was identified as one or more struts clearly separated from the vessel wall with evidence of blood speckles behind the strut. Incomplete stent apposition was classified as “persistent,” “resolved,” or “late acquired,” as previously described.
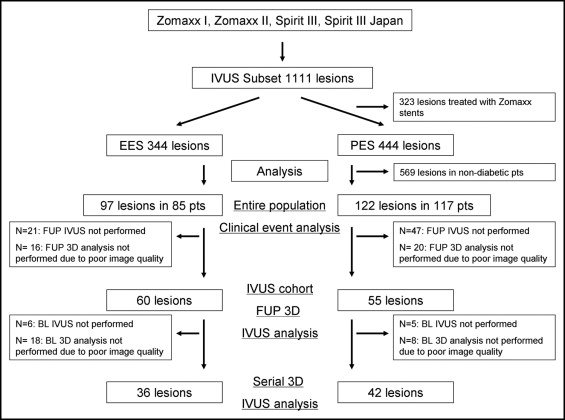
An independent clinical events committee adjudicated all events. In each trial, clinical follow-up visits were scheduled at 30 days, 6, 9, and 12 months, and annually for 5 years. In the present pooled analysis, cardiac death, myocardial infarction (defined according to the World Health Organization definition using creatine kinase and creatine kinase-MB increase ), ischemia-driven target lesion revascularization (TLR; defined as repeat percutaneous coronary intervention or coronary artery bypass grafting to the target lesion) and target vessel revascularization (TVR; defined as repeat percutaneous coronary intervention or coronary artery bypass grafting of the target vessel), and the composite end point of major adverse cardiac events (cardiac death, myocardial infarction, TLR) were evaluated for ≤2 years. Because the IVUS images were not always analyzable in patients with adverse cardiac events, these clinical events were evaluated for the entire study population involved in the present study, regardless of the IVUS image quality ( Figure 1 ).
Statistical analysis was performed using StatView, version 5.0 (SAS Institute, Cary, North Carolina). The categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables are expressed as the mean ± SD. For continuous variables, the comparisons between EESs and PESs were performed with 2-tailed, unpaired t test. Comparisons between baseline and follow-up were done using paired t test. For the subsegment analysis, data were assessed using one-way analysis of variance for comparisons within the group. Significance was assumed at p <0.05. Factors with a probability value <0.2 on univariate analysis were included in the multivariate linear regression analyses to identify the factors independently associated with the percentage of neointimal volume after stent implantation in the present study population.
Results
In the prespecified IVUS subset of these trials, we identified a total of 219 lesions in 202 patients with DM treated with either EESs or PESs, who became the candidate of clinical event analysis ( Figure 1 ). Of these cases, 68 lesions were excluded from the IVUS analysis because of the lack of follow-up IVUS images. Also, 36 lesions were excluded because of inconsistent IVUS pull back or poor IVUS image quality (16 lesions from the EES arm and 20 from the PES arm). Finally, we identified 115 lesions (60 with an EES: 36 from SPIRIT III and 24 from SPIRIT Japan; and 55 with a PES: 14 from SPIRIT III, 20 from ZoMaxx I, and 21 from ZoMaxx II) in 109 patients with DM eligible for IVUS analysis. The baseline clinical, procedural, and angiographic characteristics were well matched between the IVUS cohort and the entire study population ( Tables 1 and 2 ). In the IVUS cohort, no significant differences were found between the 2 groups, except for a longer stent length and greater percentage of diameter stenosis after stenting in the EES group.
| Variable | Entire Population (n = 202) | IVUS Cohort (n = 109) | p Value | IVUS Cohort (n = 109) | ||
|---|---|---|---|---|---|---|
| EES (n = 56) | PES (n = 53) | p Value | ||||
| Age (years) | 65.1 ± 10.0 | 64.8 ± 10.0 | 0.79 | 66.4 ± 8.6 | 63.1 ± 11.2 | 0.08 |
| Men | 123 (61%) | 69 (63%) | 0.68 | 34 (66%) | 35 (61%) | 0.56 |
| Hypertension ⁎ | 169 (84%) | 86 (79%) | 0.30 | 46 (82%) | 40 (75%) | 0.39 |
| Hyperlipidemia ⁎ | 170 (84%) | 91 (83%) | 0.88 | 47 (84%) | 44 (83%) | 0.90 |
| Current smoker | 34 (17%) | 20 (18%) | 0.74 | 8 (21%) | 12 (15%) | 0.46 |
| Previous myocardial infarction | 48 (24%) | 26 (24%) | 0.99 | 10 (18%) | 16 (30%) | 0.13 |
| Diabetes mellitus | 202 (100%) | 109 (100%) | NA | 56 (100%) | 53 (100%) | NA |
| Diabetes mellitus treatment | 0.42 | 0.90 | ||||
| Dietary therapy alone | 22 (11%) | 16 (15%) | 8 (14%) | 8 (15%) | ||
| Oral hypoglycemic agent | 145 (72%) | 79 (72%) | 40 (72%) | 39 (74%) | ||
| Insulin use | 35 (17%) | 14 (13%) | 8 (14%) | 6 (11%) | ||
| Variable | Entire Population (n = 219) | IVUS Cohort (n = 115) | p Value | IVUS Cohort (n = 115) | ||
|---|---|---|---|---|---|---|
| EES (n = 60) | PES (n = 55) | p Value | ||||
| Treated coronary artery | 0.93 | 0.79 | ||||
| Left anterior descending | 89 (41%) | 49 (43%) | 25 (42%) | 24 (44%) | ||
| Right | 75 (34%) | 39 (34%) | 22 (36%) | 17 (31%) | ||
| Left circumflex | 55 (25%) | 27 (223%) | 13 (22%) | 14 (25%) | ||
| American College of Cardiology/American Heart Association lesion type | 0.19 | 0.76 | ||||
| A-B1 | 62 (28%) | 34 (30%) | 17 (28%) | 17 (31%) | ||
| B2-C | 157 (72%) | 81 (70%) | 43 (72%) | 38 (69%) | ||
| Procedural characteristics | ||||||
| Stent length (mm) | 23.4 ± 10.6 | 23.5 ± 9.5 | 0.93 | 24.6 ± 10.0 | 20.2 ± 5.8 | 0.02 |
| Stent diameter (mm) | 3.0 ± 0.4 | 3.0 ± 0.4 | 0.73 | 3.0 ± 0.4 | 3.0 ± 0.3 | 0.97 |
| After dilation | 115 (53%) | 59 (51%) | 0.83 | 33 (55%) | 26 (47%) | 0.41 |
| Maximum inflation pressure (atm) | 15.5 ± 3.0 | 15.6 ± 3.3 | 0.75 | 16.0 ± 3.0 | 15.9 ± 3.0 | 0.89 |
| Quantitative angiographic analysis | ||||||
| Lesion length (mm) | 14.9 ± 5.9 | 15.5 ± 6.2 | 0.39 | 15.1 ± 5.8 | 15.9 ± 6.6 | 0.49 |
| Preprocedural reference diameter (mm) | 2.7 ± 0.5 | 2.7 ± 0.4 | 0.85 | 2.7 ± 0.4 | 2.7 ± 0.4 | 0.26 |
| Minimum lumen diameter after stenting (mm) | 2.7 ± 0.4 | 2.7 ± 0.4 | 0.84 | 2.7 ± 0.4 | 2.7 ± 0.4 | 0.56 |
| Diameter stenosis after stenting (%) | 2.8 ± 8.9 | 2.1 ± 8.2 | 0.49 | −0.0 ± 8.6 | 4.6 ± 7.0 | 0.002 |
The IVUS results from the 115 lesions available for follow-up IVUS analysis revealed that EESs had significantly less neointima and maximum percentage of cross-sectional narrowing than PES ( Table 3 ). The EES group had only 1 case of severe narrowing but the PES group had 6 cases (p = 0.05). The predefined subsegment analysis revealed that EESs had greater neointimal suppression than did the PESs throughout the stented segment ( Figure 2 ). Also, among the subsegments (stent edges vs stent body), no difference was seen in neointimal suppression within each group ( Figure 2 ). After adjustment for confounding parameters, a multivariate linear regression model showed that treatment with EESs (regression coefficient 6.86, p = 0.0003) and an increase in the percentage of diameter stenosis after stenting (regression coefficient 0.27, p = 0.02) were independently associated with the decrease in the percentage of neointimal volume in these patients.
| Variable | EES (n = 60) | PES (n = 55) | p Value |
|---|---|---|---|
| Neointimal volume index (mm 3 /mm) | 0.43 ± 0.46 | 0.83 ± 0.86 | 0.002 |
| Neointimal volume (%) | 7.2 ± 7.1 | 11.7 ± 11.0 | 0.01 |
| Neointimal thickness (mm) | 0.05 ± 0.05 | 0.10 ± 0.10 | 0.003 |
| Maximum percentage of cross-sectional narrowing (%) | 22.5 ± 16.3 | 29.4 ± 19.2 | 0.04 |




