Cilotax stent is a new type of drug-eluting stent (DES) designed to increase the antirestenotic performance of the paclitaxel-eluting stent and decrease the risk of stent thrombosis by the incorporation of cilostazol. Therefore, we investigated the safety and efficacy of Cilotax dual DESs and compared their performance to that of paclitaxel-eluting Taxus Liberte. Patients undergoing percutaneous coronary intervention for de novo coronary artery lesions at 2 centers in Korea were randomized to receive Cilotax (n = 55) or Taxus Liberte (n = 56) stents. The primary end point was in-segment late loss at 8 months. The 2 groups had similar baseline characteristics. Cilotax stent was not inferior to Taxus Liberte stent as determined by in-segment late loss (0.28 ± 0.30 vs 0.42 ± 0.45 mm, difference −0.14, 95% confidence interval −0.27 to −0.01, 1-sided p = 0.028 for noninferiority). In-stent late loss was significantly lower in the Cilotax than in the Taxus Liberte group (0.22 ± 0.31 vs 0.50 ± 0.55 mm, p = 0.002). Although in-segment restenosis rate did not differ significantly between the 2 groups (3.8% vs 10.9%, respectively, p = 0.271), in-stent restenosis rate was significantly lower in the Cilotax stent group (0% vs 10.9%, p = 0.027). There was no stent thrombosis at 8 months in either group. Rates of death, myocardial infarction, and any target lesion revascularization at 8 months were 0%, 0%, and 1.9%, respectively, in the Cilotax group and 1.8%, 0% and 3.6%, respectively, in the Taxus Liberte group. In conclusion, the Cilotax stent was safe and effective in decreasing late loss, indicating that this stent represents a promising new type of DES system.
Drug-eluting stents (DESs) have been widely adopted as the most effective antirestenotic strategy in coronary intervention. Despite their remarkable success, restenosis remains a significant problem for high-risk patients. Although DESs prevent restenosis by inhibiting neointimal hyperplasia, they also delay endothelialization, resulting in a more prolonged risk of stent thrombosis. In current DES systems, stents are coated with rapamycin derivatives or paclitaxel as the primary antirestenotic agents. However, restenosis results from multiple mechanisms, and late stent thrombosis remains a serious complication of current DES therapies. Dual DESs may allow differential targeting of restenosis and thrombosis with potential synergy and less toxicity. Cilostazol is a potent inhibitor of phosphodiesterase that has an antiplatelet effect similar to that of ticlopidine. Moreover, cilostazol has antiproliferative activity against cultured vascular smooth muscle cells and has been demonstrated to decrease restenosis after placement of bare metal stents or DESs. The Cilotax stent was designed to increase the antirestenotic performance of paclitaxel and decrease the risk of stent thrombosis by incorporating cilostazol. We have compared the safety and efficacy of the dual DES Cilotax with a commercially available standard paclitaxel-eluting stent in native coronary artery lesions.
Methods
From February 2008 through October 2009, 111 consecutive patients ≥18 years of age with documented ischemia at 2 centers in Korea were deemed eligible ( Figure 1 ). All patients had de novo native coronary artery lesions with target lesion diameter stenosis >50%, reference vessel 2.5 to 3.5 mm, and lesion length <20 mm by visual estimation. Exclusion criteria included left main coronary artery disease (diameter stenosis >50%), ostial lesion, planned bifurcation stenting in the side branch, acute myocardial infarction, left ventricular ejection fraction <30%, contraindication to aspirin and clopidogrel, and inability to follow the protocol. The trial protocol was approved by the ethics committee or institutional review board at each site, and all participants provided written informed consent.
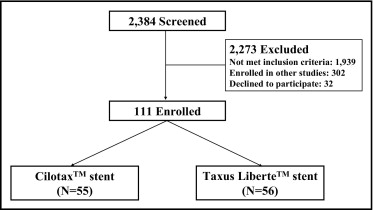
Eligible patients were randomized to receive Cilotax stents (Cardiotec Co. Ltd, Seoul, Korea) or Taxus Liberte stents (Boston Scientific Corporation, Natick, Massachusetts) with randomization concealed using a central interactive Web response service. Patients, but not investigators, were unaware of treatment assignment.
Stents were implanted according to standard techniques. Patients were pretreated with aspirin (100 to 200 mg) and clopidogrel (300 mg). During the procedure, patients received a bolus of heparin 100 U/kg, with a repeat bolus of 2,000 U to maintain an activated clotting time ≥300 seconds. Patients were discharged when clinically stable according to local practice.
Patients were prescribed aspirin (100 to 200 mg/day) indefinitely and clopidogrel (75 mg/day) for ≥12 months. Clinical evaluations were performed at time of hospital discharge and at office visits after 1, 4, 6, and 8 months with routine angiographic follow-up recommended at 8 months. All demographic, clinical, and procedural characteristics were prospectively entered into the Web-based database, and all adverse cardiac events including death, myocardial infarction, repeat revascularization, and stent thrombosis were recorded.
Coronary angiograms were sent to the core laboratory at the CardioVascular Research Foundation and were independently analyzed by experienced angiographers unaware of treatment assignment and study goal. Percent diameter stenosis, minimal lumen diameter, and reference diameter were measured using an on-line quantitative angiographic analysis system (CASS 5.7, Pie Medical Imaging, Maastricht, The Netherlands) before predilation, after the stenting procedure, and at follow-up. Angiographic measurements were made during diastole after intracoronary nitroglycerin administration using a guiding catheter to calibrate magnification. Single matched views with worst diameter stenosis were compared.
Primary end point was in-segment late loss at 8 months. Secondary end points included in-stent late loss, diameter stenosis, binary restenosis, target lesion revascularization, stent thrombosis, and major adverse cardiac events. Deaths that could not be classified were considered cardiac. Myocardial infarction was defined as clinical symptoms or occurrence of electrocardiographic changes accompanied by a new increase of creatine kinase-MB to >3 times the upper limit of normal. Stent thrombosis was classified by the Academic Research Consortium definition as definite (presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion) or probable (unexplained deaths within 30 days after the procedure or acute myocardial infarction involving the target vessel territory without angiographic confirmation) and as acute (<24 hours after procedure), subacute (1 to 30 days after procedure), or late (>30 days after procedure). Procedural success was defined as residual diameter stenosis <30% and a final Thrombolysis in Myocardial Infarction flow of grade 3.
The clinical events committee independently reviewed and adjudicated all major clinical events without information on the treatment assignment of individual patients.
Continuous variables are expressed as mean ± SD, and categorical variables are presented as frequencies. The study protocol was designed to assess the noninferiority of Cilotax stents compared to Taxus Liberte stents for the primary end point of in-segment late loss. Sample size calculation was based on a margin of noninferiority for in-segment late loss of 0.2 mm and SD of comparator of 0.5 mm. We calculated that 50 patients per group were needed to demonstrate noninferiority of the Cilotax stent with a statistical power of 80%. Expecting that up to 10% of patients would not return for follow-up coronary angiography, we sought to enroll ≥110 patients (55 patients in each arm).
All analyses were performed according to the intention-to-treat principle. Continuous variables were compared using the 2-sample t test. Categorical variables were compared using chi-square test or Fisher’s exact test. Noninferiority for in-segment late loss would be declared if the upper limit of the 1-sided 95% confidence interval of the difference was ≤0.2 mm. Superiority testing was performed after demonstration of noninferiority for the primary end points and for all other secondary end points using a 2-sided alpha value equal to 0.05. All p values are 2-sided apart from noninferiority testing of the primary end point for comparison between groups.
Results
Baseline clinical and angiographic characteristics were similar in the 2 study groups ( Table 1 ). Median patient age was 63 years (range 34 to 85), 70.3% of patients were men, and 28.8% had diabetes mellitus. Lesion characteristics and treated vessel distribution were also similar in the 2 groups ( Table 1 ). More than 80% of target lesions were type A/B1 with a mean lesion length of 13.54 ± 4.40 mm. Stent lengths per lesion were significantly longer in the Cilotax than in the Taxus Liberte stent group (20.42 ± 3.27 vs 22.14 ± 3.57 mm, p = 0.009). Stent implantation was successful in all patients.
| Characteristics | Cilotax (n = 55) | Taxus Liberte (n = 56) | p Value |
|---|---|---|---|
| Age (years) | 61.31 ± 8.68 | 60.54 ± 8.86 | 0.643 |
| Men/women | 39/16 | 39/17 | 0.884 |
| Body mass index (kg/m 2 ) | 25.1 ± 2.6 | 25.2 ± 2.7 | 0.862 |
| Current smoker | 17 (31%) | 14 (25%) | 0.488 |
| Diabetes mellitus | 16 (29%) | 16 (29%) | 0.952 |
| Hypercholesterolemia (>200 mg/dl) | 28 (51%) | 29 (52%) | 0.926 |
| Hypertension | 33 (60%) | 34 (61%) | 0.940 |
| Clinical presentation | |||
| Stable angina pectoris | 30 (55%) | 34 (61%) | 0.789 |
| Unstable angina pectoris | 18 (33%) | 15 (27%) | |
| Recent myocardial infarction | 1 (2%) | 0 (0%) | |
| Silent myocardial ischemia | 6 (11%) | 7 (13%) | |
| Previous myocardial infarction | 2 (4%) | 4 (7%) | 0.679 |
| Previous percutaneous intervention | 7 (13%) | 3 (5%) | 0.203 |
| Left ventricular ejection fraction (%) | 60.1 ± 5.5 | 60.9 ± 4.5 | 0.396 |
| Target coronary artery | |||
| Left anterior descending | 20 (36%) | 23 (41%) | 0.842 |
| Left circumflex | 15 (27%) | 13 (23%) | |
| Right | 20 (36%) | 20 (36%) | |
| Type of lesions | 0.097 | ||
| A/B1 | 44 (80%) | 51 (91%) | |
| B2/C | 11 (20%) | 5 (9%) | |
| Procedural characteristics | |||
| Balloon-to-artery ratio | 1.13 ± 0.17 | 1.10 ± 0.10 | 0.454 |
| Maximum balloon pressure (atm) | 17.91 ± 3.58 | 17.62 ± 3.92 | 0.769 |
| Stents per lesion | 1.000 | ||
| 1 | 54 (98.2%) | 54 (96.4%) | |
| 2 | 1 (1.8%) | 2 (3.6%) | |
| Stented length per lesion (mm) | 20.42 ± 3.27 | 22.14 ± 3.57 | 0.009 |
| Multivessel coronary disease | 16 (29.1%) | 12 (21.4%) | 0.353 |
Quantitative coronary angiographic results are presented in Table 2 . Angiographic follow-up was performed in 96.4% of patients (53 of 55) in the Cilotax group and 98.2% (55 of 56) in the Taxus Liberte group. At baseline, reference vessel diameter and pre- and postintervention minimal lumen diameters and acute gain were similar in the 2 groups. At 8-month follow-up, in-segment late loss tended to be lower for Cilotax than for Taxus Liberte stents, reaching the primary end point of noninferiority (0.28 ± 0.30 vs 0.42 ± 0.45 mm, difference, −0.14, 95% confidence interval −0.27 to −0.01, 1-sided p = 0.028 for noninferiority). Late loss within the stent was significantly lower in the Cilotax than in the Taxus Liberte stent group (0.22 ± 0.31 vs 0.50 ± 0.55 mm, p = 0.002), but late loss at the proximal and distal edges did not differ significantly between the 2 groups. Rates of in-segment restenosis did not differ significantly between the Cilotax and Taxus Liberte groups (3.8% vs 10.9%, p = 0.271), and target lesion revascularization was required in 3 patients (2.7%) overall. In-stent restenosis rate was significantly lower in the Cilotax than in the Taxus Liberte group (0% vs 10.9%, p = 0.027).
| Characteristics | Cilotax (n = 55) | Taxus Liberte (n = 56) | p Value |
|---|---|---|---|
| Lesion length (mm) | 13.08 ± 3.93 | 13.99 ± 4.81 | 0.281 |
| Reference vessel diameter (mm) | |||
| Before procedure | 2.96 ± 0.31 | 3.05 ± 0.32 | 0.158 |
| After procedure | 2.99 ± 0.31 | 3.06 ± 0.31 | 0.279 |
| At follow-up | 2.96 ± 0.30 | 3.05 ± 0.32 | 0.144 |
| Minimal lumen diameter (mm) | |||
| In segment | |||
| Before procedure | 0.96 ± 0.38 | 0.96 ± 0.32 | 0.990 |
| After procedure | 2.38 ± 0.41 | 2.51 ± 0.43 | 0.120 |
| At follow-up | 2.12 ± 0.48 | 2.09 ± 0.58 | 0.819 |
| In stent | |||
| After procedure | 2.69 ± 0.38 | 2.78 ± 0.36 | 0.205 |
| At follow-up | 2.48 ± 0.42 | 2.29 ± 0.64 | 0.068 |
| Diameter stenosis (%) | |||
| In segment | |||
| Before procedure | 67.7 ± 11.7 | 68.4 ± 9.8 | 0.745 |
| After procedure | 20.7 ± 7.7 | 18.2 ± 8.9 | 0.120 |
| At follow-up | 28.9 ± 11.3 | 31.7 ± 16.0 | 0.295 |
| In stent | |||
| After procedure | 10.2 ± 7.0 | 9.1 ± 6.2 | 0.365 |
| At follow-up | 16.5 ± 9.8 | 25.3 ± 19.0 | 0.003 |
| Acute gain (mm) | |||
| In segment | 1.42 ± 0.45 | 1.54 ± 0.43 | 0.140 |
| In stent | 1.73 ± 0.45 | 1.82 ± 0.39 | 0.269 |
| Late loss (mm) | |||
| In segment | 0.28 ± 0.30 | 0.42 ± 0.45 | 0.056 |
| In stent | 0.22 ± 0.31 | 0.50 ± 0.55 | 0.002 |
| Proximal edge | 0.35 ± 0.49 | 0.43 ± 0.43 | 0.338 |
| Distal edge | 0.18 ± 0.30 | 0.17 ± 0.27 | 0.799 |
| Restenosis | |||
| In segment | 2 (3.8%) | 6 (10.9%) | 0.271 |
| In stent | 0 (0%) | 6 (10.9%) | 0.027 |
| Proximal edge | 2 (3.8%) | 1 (1.8%) | 0.615 |
| Distal edge | 1 (1.9%) | 0 (0%) | 0.491 |
| Pattern of restenosis | |||
| Focal edge | 2 (3.6%) | 1 (1.8%) | 0.175 |
| Multifocal | 0 (0%) | 1 (1.8%) | |
| Diffuse proliferative | 0 (0%) | 4 (7.1%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


