Although electrocardiography is frequently used as an initial test to detect or rule out previous myocardial infarction (MI), the diagnostic performance of commonly used electrocardiographic scoring systems is not well described. We aimed to determine the diagnostic accuracy of (1) the Universal Definition, (2) Minnesota ECG Code (MC), (3) Selvester QRS Score, and (4) assessment by cardiologists using late gadolinium enhancement cardiovascular magnetic resonance imaging as the reference standard. Additionally, the effect of electrocardiographic patterns and infarct characteristics on detecting previous MI was evaluated. The 3-month follow-up electrocardiograms of 78 patients with first-time reperfused ST elevation MI were pooled with electrocardiograms of 36 healthy controls. All 114 electrocardiograms were randomly analyzed, blinded to clinical and LGE-CMR data. The sensitivity of the Universal Definition, MC, Selvester QRS Score, and cardiologists to detect previous MI was 33%, 79%, 90%, and 67%, respectively; specificity 97%, 72%, 31%, and 89%, respectively; diagnostic accuracy 54%, 77%, 71%, and 74%, respectively. Probability of detecting MI by cardiologists increased with an increasing number (odds ratio [OR] 2.00, 95% confidence interval [CI] 1.30 to 3.09), width (OR 1.02, 95% CI 1.01 to 1.03), and depth (OR 1.16, 95% CI 1.07 to 1.27) of Q waves as well as increasing infarct size (OR 1.15, 95% CI 1.06 to 1.25) and transmurality (OR 1.05, 95% CI 1.01 to 1.08; p <0.05 for all). The time-consuming MC and rapid visual assessment by cardiologists achieved the best and similar diagnostic accuracies to detect previous MI. The diagnostic performance of all 4 electrocardiographic scoring systems was modest and related to the number, depth, and width of Q waves as well as increasing infarct size and transmurality. In conclusion, the exclusion of a previous MI based solely on electrocardiographic findings should be done with caution. Future studies are needed to define which patients should be referred to additional diagnostic testing.
Although the electrocardiogram (ECG) is frequently used as an initial test to detect or rule out previous myocardial infarction (MI), the diagnostic performance of commonly used electrocardiographic scoring systems is not well described. The purpose of the present study was therefore to evaluate the diagnostic performance of the ECG criteria according to (1) the current Universal Definition (UD) for the detection of previous MI, (2) the Minnesota ECG Code (MC), (3) the Selvester QRS Score, and (4) the subjective judgment by cardiologists in a homogenous population of patients 3 months after a first-time reperfused ST elevation myocardial infarction (STEMI). Additionally, we investigated the effect of different electrocardiographic patterns and infarct characteristics, as assessed by late gadolinium enhancement cardiovascular magnetic resonance imaging (LGE-CMR), on the electrocardiographic detection of previous MI by the 4 scoring systems.
Methods
We investigated the diagnostic performance of 4 electrocardiographic scoring systems in a homogenous group of patients with previous MI, pooled with a cohort of healthy controls. Consecutive patients with a first STEMI referred to our institution from 2006 to 2008 for urgent coronary angiography and percutaneous coronary intervention were prospectively enrolled. The definition of STEMI was based on a consensus document that includes an appropriate increase and decrease in cardiac biomarkers and electrocardiographic changes indicative of new ischemia. All patients underwent ECG and CMR during admission and at 3-month follow-up. During the study period, 36 consecutive subjects without structural heart disease were enrolled from a large hospital database to serve as a control group and determine specificity. These subjects were healthy volunteers or analyzed for various reasons. All were finally diagnosed as without a cardiac disease, and none had evidence of myocardial scar (on LGE-CMR) or significant coronary artery disease (normal invasive coronary angiography, coronary computed tomography, or exercise treadmill test). Exclusion criteria included age <18 years, a history of previous MI, and regular contraindications for CMR. The presence of a left or right bundle branch block was not an exclusion criterion. Our local Institutional Review Board approved the study and informed consent was obtained from all patients before testing.
A standard 12-lead follow-up ECG was performed at a mean of 103 ± 15 days after admission, using a General Electric MAC 5500 recorder (Freiburg, Germany), and scanned into a software environment. Right-sided or posterior precordial electrocardiographic leads were not available. Quantitative electrocardiographic analysis was performed by 2 investigators, blinded to clinical and CMR data. The electrocardiogram was magnified to 400% and general information such as the heart rhythm, frequency, conduction times, and presence of left ventricular (LV) hypertrophy (according to the Sokolow-Lyon or Cornell voltage criteria) was determined. After magnification, the height and width of Q, R, and S waves in all 12 leads were measured with Cardio Calipers (version 3.3, Iconico Inc., New York) and used to calculate R/Q and R/S ratio. The surface of the Q and R waves was calculated by multiplying the height and width. ST elevation and depression were measured and the T-wave direction was determined. Measurements were displayed in millimeters, with 1 mm corresponding to 40 ms for width and to 0.1 mV for height or depth. Using these quantitative measurements, the presence or absence of MI was determined according to the UD, MC, and Selvester QRS Score for detecting previous MI.
An MI was deemed present according to the UD of previous MI in the presence of (1) any Q wave in leads V 2 to V 3 of ≥0.02 second or a QS complex in leads V 2 and V 3 , (2) Q wave of ≥0.03 second and ≥0.1 mV deep or a QS complex in leads I, III, aVL, aVF, or V 4 to V 6 in any 2 leads of a contiguous lead grouping (I, aVL, and V 6 ; V 4 to V 6 ; II, III, and aVF), or (3) an R wave ≥0.04 second in V 1 to V 2 and R/S ≥1 with a concordant positive T wave in the absence of a conduction defect.
The diagnostic performance of the MC to detect previous MI was determined using all codes for abnormal Q and QS patterns (i.e., codes 1-1-1 to 1-3-6). Additionally, we evaluated the effect of inclusion or exclusion of weaker Q, ST, and T abnormalities on the diagnostic accuracy of the MC. The presence of major or intermediate Q waves (i.e., codes 1-1-x to 1-2-x) was considered a sign of “definite MI”, additional minor Q waves (i.e., codes 1-3-x) in combination with major ST (i.e., codes 4-1 or 4-2) and T abnormalities (i.e., codes 5-1 or 5-2) were categorized as “probable MI”, and all Q codes for abnormal Q and QS patterns (i.e., codes 1-1-1 to 1-3-6) were deemed “possible MI”.
The Selvester QRS Score was performed according to the detailed guide described by Loring et al. Because each point awarded on this score has been shown to represent 3% infarct size of the LV, a score of ≥1 was considered to be indicative of previous MI.
All 114 electrocardiograms were qualitatively analyzed independently and in random order by 2 cardiologists (SS and SCB), who were blinded to patient, clinical, and CMR data. The intraobserver (κ = 0.72 for both) and interobserver agreement (κ = 0.68) of electrocardiographic interpretation by cardiologists was good. Discrepancies were resolved in consensus after review by a third expert (APG). Cardiologists were asked to give a subjective judgment of the absence or presence (including location) of an MI.
If an MI was detected by any of the 4 approaches, electrocardiographic changes were compared with the infarct-related artery on coronary angiography to determine the electrocardiographic MI location. Changes in the anterior leads (i.e., I, aVL, V 1 to V 6 ) were considered congruent for the left anterior descending artery, whereas changes in nonanterior leads (i.e., a Q wave in II, III, and aVF and/or an R wave in V 1 to V 2 ) were congruent for the left circumflex (LCx) or right coronary artery.
Patients underwent follow-up CMR imaging at a mean of 104 ± 11 days (range 75 to 141 days) after admission. Images were acquired with a 1.5 T magnetic resonance imaging system (Philips Intera, Philips Medical Systems, Best, The Netherlands) equipped with a cardiac software package and 5-element phased array surface coil.
Electrocardiographic-gated cine imaging was performed for functional analysis using a multiple breath-hold steady-state free precession sequence (slice thickness 6 mm, slice gap 4 mm, repetition time/echo time 3.8/1.9 ms, flip angle 50°, field of view 350 mm, matrix 256 × 256, 22 to 25 phases per cardiac cycle) in 2-chamber, 3-chamber, and 4-chamber view and a short-axis stack covering the entire LV. LGE images were acquired 10 minutes after an administration of 0.2 mmol/kg body weight gadolinium diethylenetriaminepentaacetic acid (Magnevist, Bayer Schering, Germany), using a breath-hold 3-dimensional inversion-recovery gradient echo technique (acquired/reconstructed slice thickness 12/6 mm, average repetition time/echo time 3.9/2.4 ms, multishot [50 profiles/shot] segmented partial echo readout, flip angle 15°, field of view 400 mm, matrix 256 × 256) in short-axis, 2-chamber, and 4-chamber views. Inversion delay time was set to null signal from normal myocardium (typically 200 to 280 ms).
CMR images were analyzed independently by 2 observers, experienced in reading CMR and blinded to clinical data, using commercially available software (CAAS MRV 3.0, Pie Medical Imaging, Maastricht, The Netherlands). Endocardial and epicardial borders were manually traced on short-axis cine images, excluding the papillary muscles, in the end-diastolic and end-systolic phases to determine LV volumes, LV ejection fraction, and mass. Similarly, endocardial and epicardial borders were manually traced on the short-axis LGE images. Infarct size was measured by manually tracing hyperenhanced areas and expressed as a percentage of LV mass (interobserver agreement of κ = 0.90). Transmural extent of infarction (%) was calculated by dividing the hyperenhanced area by the total area of a predefined segment according to the American Heart Association 17-segment model.
Continuous variables with normally distributed data are expressed as mean ± SD, otherwise as median with the interquartile range (IQR). Categorical data are expressed as frequencies with percentages. The inter- and intraobserver agreement between the 2 readers was analyzed using Cohen’s kappa (κ) coefficient. Differences in categorical data were evaluated using a chi-square or Fisher’s exact tests. For continuous data, the independent t test and Mann Whitney U test were used for normally and non-normally distributed data, respectively. The sensitivities, specificities, positive predictive value (PPV) and negative predictive value, and diagnostic accuracies of the 4 approaches were calculated and compared using the McNemar test, with LGE-CMR as the reference standard.
The UD, MC, and Selvester ECG Score are, by definition, strongly related to specific electrocardiographic patterns (e.g., the presence or number of significant Q or R waves). Therefore, we only performed a univariate binary logistic regression analysis to explore the effect of these different electrocardiographic patterns on the probability of MI detection by cardiologists. Additionally, we explored the effect of infarct characteristics on accurate detection of MI by all 4 electrocardiographic scoring systems. The dependent variable in these analyses was whether the MI was detected by ECG (detected vs undetected). Because of the relatively limited number of undetected MI, it was not possible to perform a multivariate logistic regression analysis to evaluate the independent effects of specific characteristics. SPSS version 17.0 (SPSS Inc., Chicago, Illinois) was used for all statistical analyses. A 2-tailed value of p <0.05 was considered statistically significant.
Results
Patients with MI were on average older than healthy controls, more often men, and active or ex-smokers ( Table 1 ). Thrombolysis In Myocardial Infarction 3 flow after percutaneous coronary intervention was established in most patients with MI. The median time between the ECG and CMR imaging was shorter in patients than in healthy controls (i.e., 0 days [IQR −4 to 3] vs 30 days [IQR 12 to 54], respectively, p <0.001). In all patients with STEMI, hyperenhancement was visible on LGE-CMR images matching the territory of the infarct-related artery on coronary angiography. None of the patients with STEMI showed additional areas of LGE and, thus, no evidence of unrecognized previous MI.
| Variable | Patients n = 78 | Controls n = 36 | p |
|---|---|---|---|
| Age (yrs) | 59 ± 11 | 43 ± 12 | <0.001 |
| Men | 61 (78) | 20 (56) | <0.05 |
| Diabetes mellitus | 6 (8) | 1 (3) | 0.67 |
| Smoker (current or ex) | 67 (86) | 4 (11) | <0.001 |
| Hypertension | 27 (35) | 7 (19) | 0.91 |
| Hypercholesterolemia | 22 (28) | 4 (11) | 0.17 |
| Positive family history | 38 (49) | 14 (39) | 0.69 |
| Infarct-related coronary artery | |||
| Left anterior descending | 24 (31) | — | |
| Left circumflex | 11 (14) | — | |
| Right coronary | 43 (55) | — | |
| Number of narrowed coronary arteries | |||
| 1 | 38 (49) | — | |
| ≥2 | 40 (51) | — | |
| TIMI 3 | |||
| Before PCI | 6 (8) | ||
| After PCI | 69 (88) | ||
| Electrocardiogram | |||
| Post-MI (days) | 103 ± 15 | — | |
| Sinus rhythm | 77 (99) | 36 (100) | 1.00 |
| Frequency (beats/min) | 65 ± 12 | 68 ± 9 | 0.22 |
| Atrioventricular block | 0.31 | ||
| First degree | 4 (5) | 0 (0) | |
| Second or third degree | 0 (0) | 0 (0) | |
| Bundle branch block | 0.09 | ||
| Left or right bundle branch block | 0 (0) | 0 (0) | |
| Left anterior fascicular block | 2 (3) | 3 (8) | |
| Bifascicular block | 1 (1) | 3 (8) | |
| Interventricular conduction delay | 2 (3) | 3 (8) | |
| LV hypertrophy ∗ | 7 (9) | 0 (0) | 0.10 |
| Cardiovascular magnetic resonance | |||
| Post-MI (days) | 104 ± 11 | — | |
| Right ventricular involvement | 9 (12%) | — | |
| Infarct size (% of LV) | 10 (5–17) | — | |
| Infarct transmurality (%) | 55 ± 16 | — | |
| Number of infarcted segments | 8 ± 3 | — | |
| LV ejection fraction (%) | 54 ± 8 | 61 ± 5 | <0.001 |
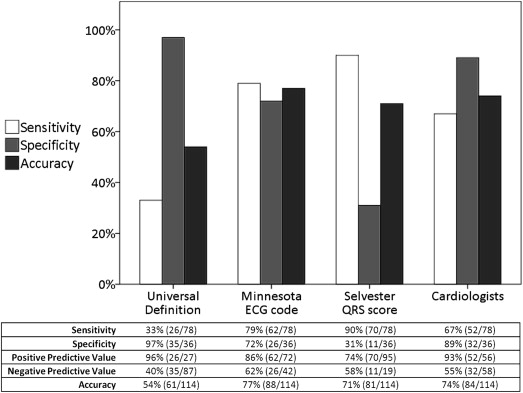
The diagnostic performance of the 4 electrocardiographic scoring systems to detect a previous MI is shown in Figure 1 . The sensitivities ranged from 33% for the UD to 90% for the Selvester QRS Score, with specificities ranging from 31% for the Selvester QRS Score to 97% for the UD. The MC and assessment by cardiologists yielded similar superior overall diagnostic accuracies (p = 0.56). The UD and assessment by cardiologists demonstrated the highest PPVs, but with low negative predictive values.