We sought to determine the significance of incomplete stent frame expansion after transcatheter aortic valve implantation with the Medtronic-CoreValve device. Incomplete coronary stent expansion is a well-described phenomenon. Transcatheter valves are mounted on stents; however, the incidence of incomplete stent expansion after transcatheter aortic valve implantation, its sequelae and predictors are poorly elucidated. The 18Fr CoreValve revalving system was used to treat anatomically and clinically suitable patients with severe calcific aortic stenosis. The postdeployment stent dimensions were measured on fluoroscopic images at multiple levels of the stent frame. Incomplete expansion at each was defined as <100% expected and complete expansion as ≥100%. These parameters were correlated to the hemodynamic and clinical end points. A total of 50 consecutive patients underwent transcatheter aortic valve implantation with the Medtronic-CoreValve at a single center from January 2007 to December 2008. For the inflow portion, incomplete expansion was seen in 54% of patients and was unrelated to the aortic valve area, peak or mean aortic valve gradients, or measures of aortic regurgitation, although it was paradoxically associated with a lower incidence of prosthesis–patient mismatch. Incomplete expansion of the constrained portion was seen in 62% of the patients and displayed a trend toward a greater incidence of aortic regurgitation grade 2 or greater, although this was rare. Incomplete expansion of the Medtronic CoreValve stent frame is common. For the most part, valvular hemodynamic function was satisfactory, regardless of the degree of expansion of the stent frame that carries it and a strategy of reluctant postdilation in the context of incomplete stent frame expansion was supported.
Incomplete coronary stent expansion is a well-described phenomenon, has a greater likelihood in calcified lesions and can result in dramatically adverse sequelae such as restenosis and stent thrombosis. Transcatheter aortic valve implantation (TAVI) has shown great promise in the treatment of severe aortic stenosis in patients regarded at high risk from conventional surgery. Transcatheter valves are mounted on stents. The Medtronic-CoreValve stented bioprosthesis (Medtronic Inc., Minneapolis, Minnesota) has a relatively long self-expanding stent frame and has the potential for incomplete expansion, particularly in the setting of severe calcific aortic stenosis. Moreover, incomplete expansion could theoretically have a bearing on the leaflet function of the valve it carries. We sought to describe this phenomenon and assess its hemodynamic and early clinical significance in patients undergoing TAVI with the CoreValve bioprosthesis.
Methods
The clinical and anatomic selection criteria were in accordance with the published investigational study for the third-generation (18Fr) CoreValve device. The patients were recruited from a single center and underwent TAVI with the CoreValve bioprosthesis by way of a transfemoral route. All patients had severe calcific aortic stenosis (aortic valve area [AVA] <1 cm 2 or body surface area-indexed AVA <0.6 cm 2 /m 2 ). They were symptomatic, considered at high risk of, or inoperable by, conventional surgery, and had annuli of 20 to 27 mm and suitable peripheral vasculature (iliofemoral diameter >6 mm). Patients with annuli of 20 to 23 mm received a 26-mm prosthesis; those with annuli of 23 to 27 mm received a 29-mm prosthesis. The device was implanted as previously described.
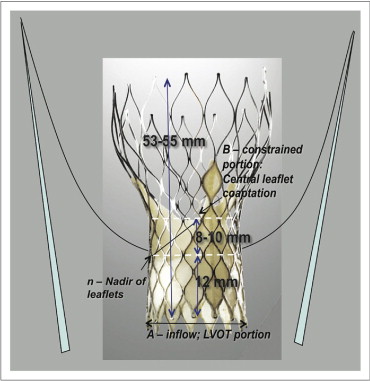
The postdeployment stent dimensions were assessed in the optimal angiographic plane for implantation, which displayed the 3 aortic valve sinuses in line and, in turn, minimized the possibility of foreshortening during measurements. The stent measurements were made at the inflow level (level A) and at the level of the most constrained portion (level B, close to the level of central coaptation of the bioprosthetic leaflets, approximately 20 to 22 mm from the inflow margin of the stent-frame; Figure 1 ). Measurements of the prosthesis were also made at the level of the annulus (a′) and 12 mm above the inflow margin of the stent frame (n), which corresponds to the nadir of the prosthetic leaflets and also the lowest point of insertion of the tissue skirt on the external surface of the stent frame. The depth of stent frame implantation from the native noncoronary cusp was measured. Using this measurement, the optimal depth of implantation was defined at 5 to 10 mm 8 . Given the 12-mm nadir of the leaflets from the inflow margin, this “optimal” implantation depth of 5 to 10 mm would correlate to the nadir lying 2 to 7 mm above the native annulus. Accurate calibration was performed retrospectively, off-line, using images obtained in the same projection during balloon predilation from immediately before CoreValve implantation, using the Nucleus valvuloplasty (NuMed Inc., Hopkinton, New York) balloon markers (20 mm apart). Severe valvular calcification was assessed qualitatively using a locally derived fluoroscopic score (see the Appendix ). The expected in vitro dimensions of the 2 sizes of prosthesis available were supplied by the manufacturer. At both level A (26 or 29 mm for 26- and 29-mm devices, respectively) and level B (22 or 24 mm for 26- and 29-mm devices, respectively), a standardized method for representation of the degree of stent expansion was defined as (measured dimension)/(expected dimension) × 100% at each level. Incomplete expansion was defined as <100%, moderate underexpansion as <90%, and gross or severe underexpansion as <80%. These were correlated to the hemodynamic end points. A comprehensive 2-dimensional and Doppler transthoracic echocardiogram was recorded with the patient in the left lateral decubitus position immediately before the procedure and before discharge. The transesophageal echocardiographic data immediately after implantation were used in 2 cases in which the transthoracic echocardiographic data were unavailable. Standard views were obtained with simultaneous electrocardiographic monitoring recorded. Second harmonic imaging (1.8 to 3.6 MHz) was used to enhance the endocardial border definition. Measurements were made using Heartlab imaging software (Agfa-Gevaert Group, Mortsel, Belgium). The hemodynamic end points included the continuity effective orifice AVA, indexed AVA, frequency of prosthesis–patient mismatch (indexed AVA ≤0.85 cm 2 /m 2 ), and peak and mean aortic valve gradients on the predischarge echocardiogram. Echocardiographic aortic regurgitation was graded semiquantitatively using an established integrative approach with parasternal long-axis view observations : none (grade 0), trivial or mild (grade 1+), moderate (grade 2+), moderate to severe (grade 3+), and severe (grade 4+). Paraprosthetic or transvalvular aortic regurgitation (AR) were determined qualitatively at each point.
Statistical analysis was done using the Statistical Package for Social Sciences software (SPSS, Chicago, Illinois). Differences were assessed using a paired sample t test for normally distributed data or Wilcoxon signed rank 2-related samples analyses for other distributions. For comparison of independent nonparametric variables, a chi-square statistic was used and for independent normally distributed variables, an independent samples t test. For correlations, Pearson’s bivariate analysis with a 2-tailed test for significance was used for parametric variables and Spearman’s rho correlation for nonparametric variables. Factors related univariately to incomplete stent expansion were studied in a multivariate binary logistic regression model, where appropriate. We assessed the influence of the degree of stent-frame expansion on the echocardiographic, fluoroscopic, and cardiac catheter parameters described in the previous paragraphs.
Results
From January 2007 to December 2008, 50 patients underwent TAVI. The baseline demographics are listed in Table 1 . There was 1 case of trivial transvalvular AR; the remainder were paravalvular. The hemodynamic data are listed in Table 2 . Incomplete stent-frame expansion at the inflow (level A) was seen in 54% of patients ( Table 3 ). In 32%, this was mild, in 20% moderate, and in 2% severe ( Figure 2 ). No significant differences were seen in the baseline variables between the patients with incomplete or complete inflow expansion. The stents with an incompletely expanded inflow tended to be positioned less deeply (p = 0.015) and were more likely to be optimally implanted (p = 0.047; Table 3 ). Incomplete stent frame expansion at the most constrained portion (level B) was seen in 62% ( Table 4 ). In 30%, this was mild, in 22% moderate, and in 10% severe. Regarding the baseline variables, only a larger annulus size was associated with an incompletely expanded stent-frame at level B (p = 0.048). Similarly, the larger 29-mm inflow device was less likely to attain the expected dimensions at the constrained portion (p = 0.006).
| Characteristic | Value |
|---|---|
| Mean age (years) | 83 ± 6 |
| Men | 24 (48%) |
| Diabetes mellitus | 9 (18%) |
| Hypertension | 21 (42%) |
| Atrial fibrillation | 14 (28%) |
| Ischemic heart disease | 23 (46%) |
| Previous myocardial infarction | 7 (14%) |
| Previous cerebrovascular accident/transient ischemic attack | 7 (14%) |
| Previous coronary artery bypass grafting | 9 (18%) |
| Previous percutaneous coronary intervention | 15 (30%) |
| Previous permanent pacemaker | 4 (8%) |
| Peripheral vascular disease | 5 (10%) |
| Dialysis | 2 (4%) |
| Creatinine >200 μmol/L | 2 (4%) |
| Any renal impairment (estimated glomerular filtration rate <50 ml/min) | 24 (48%) |
| Pulmonary disease | 16 (32%) |
| Previous malignancy | 7 (14%) |
| Active malignancy | 3 (6%) |
| Declined outright for open surgery | 17 (34%) |
| Multiple risk factors, Logistic EuroSCORE >30 | 6 (12%) |
| Severe respiratory disease | 5 (10%) |
| “Porcelain” aorta ⁎ | 3 (6%) |
| Age >90 years | 2 (4%) |
| No contractile reserve on stress echocardiogram | 1 (1%) |
| Symptoms | |
| Dyspnea | 50 (100%) |
| Angina pectoris | 34 (68%) |
| Syncope | 7 (14%) |
| Emergent case/cardiogenic shock | 1 (2%) |
| New York Heart Association class | |
| I | 0 |
| II | 24 (48%) |
| III | 22 (44%) |
| IV | 4 (8%) |
| Biplane left ventricular ejection fraction (%) | 50 ± 13 |
| ≤30% | 7 (14%) |
| ≤40% | 15 (30%) |
| ≤50% | 23 (46%) |
| Logistic EuroSCORE | 22 (13%) |
| Logistic EuroSCORE >20 | 25 (50%) |
| Peak transvalvular pressure gradient (mm Hg) | 80 ± 30 |
| Mean transvalvular pressure gradient (mm Hg) | 47 ± 19 |
| Aortic valve area (cm 2 ) | 0.7 ± 0.2 |
⁎ Severely calcified aorta preventing the possibility of aortic cross-clamping.
| Variable | Before | After | p Value |
|---|---|---|---|
| Peak aortic gradient (mm Hg) | 81 ± 30 | 16 ± 6 | <0.001 |
| Mean aortic gradient (mm Hg) | 48 ± 18 | 8 ± 3 | <0.001 |
| Aortic valve area (cm 2 ) | 0.7 ± 0.2 | 1.7 ± 0.5 | <0.001 |
| Aortic valve area indexed to body surface area (cm 2 /m 2 ) | 0.4 ± 0.1 | 1.0 ± 0.3 | <0.001 |
| Prosthetic aortic valve area indexed to body surface area (cm 2 /m 2 ) | |||
| ≤0.85 | — | 16 (32%) | — |
| ≤0.8 | — | 14 (28%) | — |
| ≤0.65 | — | 1 (2%) | — |
| AR grade | 0.196 | ||
| 0 | 14 (28%) | 8 (16%) | |
| 1 | 32 (64%) | 38 (76%) | |
| 2 | 3 (6%) | 2 (4%) | |
| 3 | 1 (2%) | 1 (2%) | |
| 4 | 0 | 1 (2%) | |
| Biplane left ventricular ejection fraction (%) | 50 ± 13 | 56 ± 9 | 0.001 |
| ≤30% | 7 (14%) | 0 | 0.008 |
| ≤40% | 15 (30%) | 6 (12%) | 0.005 |
| ≤50% | 23 (46%) | 12 (24%) | 0.005 |
| New York Heart Association class | <0.001 | ||
| I | 0 | 27 (54%) | |
| II | 24 (48%) | 16 (32%) | |
| III | 22 (44%) | 2 (4%) | |
| IV | 4 (8%) | 0 |
| Variable | Incomplete Expansion (n = 27) | Complete Expansion (n = 23) | p Value |
|---|---|---|---|
| Mean age (years) | 83 ± 6 | 83 ± 6 | 0.901 |
| Men | 15 (56%) | 9 (39%) | 0.247 |
| Body mass index (kg/m 2 ) | 25 ± 3 | 24 ± 3 | 0.283 |
| Body surface area (m 2 ) | 1.8 ± 0.2 | 1.7 ± 0.2 | 0.223 |
| Diabetes mellitus | 6 (22%) | 3 (13%) | 0.4 |
| Hypertension | 11 (41%) | 10 (43%) | 0.845 |
| Peripheral vascular disease | 2 (7%) | 3 (13%) | 0.508 |
| Severe valvular calcification | 5 (18%) | 6 (26%) | 0.520 |
| “Porcelain” aorta | 1 (4%) | 2 (9%) | 0.459 |
| Previous cardiac surgery | 7 (26%) | 2 (9%) | 0.114 |
| Aortic annulus size (mm) | 23.1 (2.0) | 23.1 (1.6) | 0.981 |
| Left ventricular outflow track size (mm) | 21.2 ± 3.0 | 21.2 ± 2.8 | 0.999 |
| Sinotubular junction size (mm) | 27.4 ± 4.7 | 28.7 ± 3.7 | 0.273 |
| Preprocedure biplane left ventricular ejection fraction (%) | 50.8 ± 13.6 | 48.4 ± 13.6 | 0.531 |
| Preprocedure aortic valve area (cm 2 ) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.718 |
| Preprocedure peak gradient (mm Hg) | 80.8 ± 25.4 | 78.3 ± 36.0 | 0.767 |
| Preprocedure mean gradient (mm Hg) | 47.7 ± 16.7 | 47.0 ± 21.1 | 0.900 |
| Inflow device (mm) | |||
| 29 | 12 (44%) | 8 (35%) | 0.487 |
| 26 | 15 (56%) | 15 (65%) | 0.487 |
| Depth of implant (cm) | 9.4 ± 3.7 | 11.7 ± 2.6 | 0.015 ⁎ |
| “Optimal” implant position | 17 (63%) | 8 (35%) | 0.047 ⁎ |
| Postdilation | 3 (11%) | 1 (4%) | 0.38 |
| Diameter of level A (mm) | 25.1 ± 2.1 | 29.3 ± 2.5 | <0.001 ⁎ |
| A, actual dimension of expected dimension (%) | 92 ± 6 | 109 ± 8 | <0.001 ⁎ |
| Variable | Incomplete Expansion (n = 31) | Complete Expansion (n = 19) | p Value |
|---|---|---|---|
| Mean age (years) | 83 ± 5 | 83 ± 7 | 0.786 |
| Men | 18 (58%) | 6 (32%) | 0.069 |
| Body mass index (kg/m) | 24 ± 3 | 25 ± 3 | 0.770 |
| Body surface area (m 2 ) | 1.8 ± 0.2 | 1.7 ± 0.2 | 0.177 |
| Diabetes mellitus | 4 (21%) | 5 (16%) | 0.660 |
| Hypertension | 9 (47%) | 12 (39%) | 0.547 |
| Peripheral vascular disease | 5 (16%) | 0 (0) | 0.065 |
| Severe valvular calcification | 9 (29%) | 2 (11%) | 0.125 |
| “Porcelain” aorta | 1 (3%) | 2 (11%) | 0.291 |
| Previous cardiac surgery | 8 (26%) | 1 (5%) | 0.066 |
| Aortic annulus size (mm) | 23.4 ± 2.1 | 22.5 ± 1.1 | 0.048 ⁎ |
| Left ventricular outflow track size (mm) | 21.6 ± 3.2 | 20.6 ± 0.2 | 0.211 |
| Sinotubular junction size (mm) | 28.7 ± 4.5 | 27.0 ± 3.9 | 0.189 |
| Preprocedure biplane left ventricular ejection fraction procedure (%) | 49.0 (13.9) | 50.7 (13.1) | 0.667 |
| Preprocedure aortic valve area (cm 2 ) | 0.7 (0.2) | 0.7 (0.3) | 0.662 |
| Preprocedure peak gradient (mm Hg) | 81.7 (30.7) | 76.4 (30.5) | 0.560 |
| Preprocedure mean gradient (mm Hg) | 48.4 (18.4) | 45.7 (19.5) | 0.621 |
| Inflow device (mm) | |||
| 29 | 17 (55%) | 3 (16%) | 0.006 ⁎ |
| 26 | 14 (45%) | 16 (84%) | 0.006 ⁎ |
| Depth of implant (cm) | 10.6 ± 3.4 | 10.3 ± 3.4 | 0.698 |
| “Optimal” implant position | 18 (58%) | 7 (37%) | 0.145 |
| Postdilation | 3 (10%) | 1 (5%) | 0.066 |
| Diameter of B (mm) | 20.6 ± 1.8 | 23.8 ± 1.4 | <0.001 ⁎ |
| B, actual dimension of expected dimension (%) | 89 ± 89 | 107 ± 5 | <0.001 ⁎ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


