Obesity is independently associated with left ventricular (LV) hypertrophy and thus may be an important modifier of the hypertrophic cardiomyopathy (HC) phenotype. We examined if obesity modifies the clinical presentation, LV morphology, outflow hemodynamics, and exercise tolerance in HC. In this cross-sectional study, 88 obese (body mass index [BMI] ≥30 kg/m 2 ) and 154 nonobese (BMI <30 kg/m 2 ) patients from the Johns Hopkins HC clinic were compared with respect to a variety of clinical and LV echocardiographic measurements. Obese patients (36.4%) were more likely to report exertional dyspnea (p = 0.04) and chest pain (p = 0.002) and had greater prevalence of hypertension (p = 0.008). LV posterior wall thickness (p = 0.01) but not the septal wall (p ≥0.21) was significantly greater in obese patients, resulting in an increased LV mass index (p = 0.003). No significant differences in LV systolic and diastolic functions were observed, but obesity was associated with higher LV stroke volume (p = 0.03), inducible LV outflow tract gradients (p = 0.045), and chance of developing LV outflow tract obstruction during stress (p = 0.035). In multivariate analysis, BMI was associated with increased posterior (but not septal) wall thickness (β = 0.15, p = 0.02) and LV mass index (β = 0.18, p = 0.005), particularly in those with hypertension. Obesity was also associated with reduced exercise time and functional capacity, and BMI independently correlated with reduced exercise tolerance. In conclusion, obesity is associated with larger LV mass, worse symptoms, lower exercise tolerance, and labile obstructive hemodynamics in HC. The association with increased outflow tract gradients has particular importance as contribution of obesity to the pressure gradients may influence clinical decisions in labile obstructive HC.
Hypertrophic cardiomyopathy (HC) is the most common genetic cardiovascular disease affecting about 1 in 500 persons in the general population and manifesting as left ventricular (LV) hypertrophy, life-threatening arrhythmias, and heart failure. Obesity has become the most prevalent chronic medical condition in western countries and has a major influence on the development of cardiac disease. Despite the large body of evidence indicating an obesity-LV hypertrophy link, there is a glaring lack of investigation into the potential influence of obesity on LV hypertrophy in HC. Recent guidelines recommend aggressive modification of common cardiovascular risk factors, including obesity, in patients with HC. However, beyond the purpose of reducing global cardiovascular risk, a specific rationale for pursuing aggressive weight loss in HC is lacking. A single intriguing case report wherein substantial weight loss resulted in a decrease in LV wall thickness in a patient with HC suggests that some of the LV hypertrophy in HC could be modulated by obesity. Wall thickness is not only a cardinal anatomic feature in HC but may be responsible for LV outflow tract obstruction and heart failure–related symptoms, it counts as a risk factor when assessing a patient’s risk profile for ventricular arrhythmias, and factors into a decision to implant a defibrillator. We hypothesized that obesity would be associated with worse LV hypertrophy in HC and thereby potentially affect LV function and exercise capacity. Accordingly, the aims of the study were to analyze the relation of obesity to clinical presentation, LV structure, function, and hemodynamics as determined by echocardiography and functional capacity as determined by treadmill exercise.
Methods
This is a cross-sectional analysis of a prospectively generated database of 258 consecutive patients with HC at their first visit to the Johns Hopkins HC clinic from February 2005 to July 2012. Data were collected for patients fulfilling the criteria for a diagnosis of HC, defined as unexplained LV hypertrophy with a maximal wall thickness >15 mm in the absence of other cardiac or systemic condition capable of producing a similar degree of LV hypertrophy. Features suggestive of HC such as systolic anterior movement (SAM) of the mitral valve, LV outflow tract obstruction, and family history of HC were also taken into account for confirming the diagnosis. Patients diagnosed with apical HC (n = 10) or with previous septal myectomy or alcohol ablation (n = 6) were excluded from the present analysis. This study was approved by the Johns Hopkins Institutional Review Board.
Height and weight were used to calculate body mass index (BMI, kg/m 2 ), and obesity was defined as a BMI ≥30 kg/m 2 . Clinical data including symptoms, co-morbidities, medications, and family history of HC, sudden cardiac death, and coronary artery disease were ascertained by the examining physician. Systolic and diastolic blood pressures were measured during clinical examination. Hypertension was defined as blood pressure ≥130/85 at initial examination or self-reported hypertension with need for antihypertensive medication. Participants who had never smoked >100 cigarettes in their life were considered as nonsmokers. Diabetes mellitus was defined as self-reported diabetes or the use of antidiabetic drugs. Dyslipidemia was defined as self-reported dyslipidemia with need for lipid-lowering medication. Self-reported functional capacity was classified according to the New York Heart Association classification.
All echocardiographies were performed using a Vivid 7 cardiac ultrasound machine (GE Healthcare, Milwaukee, Wisconsin) with a 3.5-MHz transducer and following a standardized protocol.
The LV linear dimensions were measured from a parasternal long-axis view. End-diastolic wall thickness was measured at the basal segment and mid segment of the interventricular septum and at the basal segment of the posterior wall as recommended. Maximal septal wall thickness was divided by basal posterior wall thickness to calculate septal-to-posterior wall thickness ratio, a parameter used to estimate asymmetric septal LV hypertrophy. LV end-diastolic and end-systolic volumes were calculated using the biplane Simpson method and stroke volume as the difference between the two. The monoplane Simpson method was used to measure the left atrial volume in the end-systole in apical 4-chamber view, which was then indexed to body surface area. Relative wall thickness, LV mass, and ejection fraction were calculated with formulas recommended by the American Society of Echocardiography. To adjust for the influence of growth without eliminating the impact of excess adiposity, LV mass and volumes were indexed to height in meters to the allometric power of 2.7, as previously suggested. From the apical window, pulsed Doppler sample was placed at the mitral valve leaflet tips and the peak velocity of early diastolic mitral inflow (E) and late diastolic mitral inflow (A) were measured and their ratio (E/A ratio) calculated. Tissue Doppler early diastolic velocity of the septal mitral annulus (Em) was recorded and the E/Em ratio calculated.
LV outflow tract pressure gradients were measured in the most optimal apical view by continuous-wave Doppler echocardiography under resting conditions and during provocative maneuvers including Valsalva, treadmill exercise, and/or amyl nitrite inhalation to elicit latent obstruction. Resting and maximal inducible LV pressure gradients were used to classify patients with HC into nonobstructive, labile obstructive, or obstructive according to recent guidelines. LV outflow tract diameter was determined at rest at the onset of systole by measuring the minimal distance between the left side of the interventricular septum and the initial echo of the anterior mitral leaflet in the parasternal long-axis view. SAM was evaluated by B-mode echocardiographic images and defined as incomplete or complete on the basis of the absence or presence of contact between the mitral leaflet and the septum, respectively.
Deformation analysis based on speckle tracking was performed off-line using dedicated GE EchoPac software, v. 7.1.1 (GE Ultrasound, Norway) for estimating global longitudinal systolic strain. Three consecutive cardiac cycles with a frame rate of 60 to 90/s were recorded. The endocardial border of the LV cavity was traced manually from a still frame image and automatically tracked throughout the cardiac cycle by the software. Poorly tracking segments or images that could not be optimized were discarded and not considered for the analysis.
Patients underwent treadmill exercise testing unless excluded per the conditions: unable to walk on the treadmill, blood pressure >220/120 mm Hg, unstable angina, recent myocardial infarction (<1 week), decompensated heart failure, and active atrial arrhythmia (heart rate at rest >120 beats/min). A standard Bruce protocol was implemented unless patients reported very low exercise tolerance, in which case a modified Bruce protocol was used. Data were collected regarding exercise capacity (expressed as metabolic equivalents [METs] or exercise time in seconds), percentage exercise predicted heart rate (calculated as peak exercise heart rate divided by maximum predicted heart rate and multiplied by 100), systolic and diastolic blood pressures, arrhythmias, and symptoms at peak exercise.
All statistical analyses were performed using the SAS package (version 9.3, SAS Institute Inc., Cary, North Carolina). Continuous variables are presented as mean ± SD and categorical variables as proportions. Comparisons between obese and nonobese patients were performed by Student’s t tests or chi-square tests as appropriate. Simple correlations were estimated using Spearman’s nonparametric rank correlation coefficient (r s ). Univariate and multivariate linear regressions were used to assess the independent association of BMI with functional capacity (METs) and LV structural parameters (maximal septal wall thickness, posterior wall thickness, and LV mass index). Hypertension (as a dichotomous variable) and LV outflow tract obstruction (as a categorical variable, where 0 = nonobstructive, 1 = labile obstructive, and 2 = obstructive) were included as covariates. Standardized coefficients were also calculated to facilitate comparison of regression coefficients in the same model. In all models, the interaction between BMI and other covariates was tested. One-way analysis of variance with the post hoc Tukey-Kramer multiple range test was used to compare METs, septal and posterior wall thicknesses, and LV mass index in patients with and without obesity and hypertension after adjusting for LV outflow tract obstruction. Statistical significance was set at p ≤0.05.
Results
The study population consisted of 242 patients with HC and was divided into 2 groups on the basis of a BMI ≥30 kg/m 2 versus <30 kg/m 2 . Demographic and clinical characteristics of the 2 groups are listed in Table 1 . Prevalence of obesity in our sample was 36.4%, and 48 obese patients (19.8%) had severe obesity (BMI ≥35 kg/m 2 ). There were no significant differences in age, gender, race, and family history between the groups. There was a trend for greater prevalence of common cardiovascular risk factors in obese patients, but this was significant only for hypertension. Accordingly, the use of angiotensin-converting enzyme inhibitors was greater in obese patients, but there was no statistical difference in systolic blood pressure. At the time of their initial clinical presentation, obese patients showed greater prevalence of chest pain, dyspnea, and limitation in physical activity (New York Heart Association class ≥II) and no significant differences in syncope and atrial fibrillation ( Table 1 ).
| Variable | BMI (kg/m 2 ) | p | |
|---|---|---|---|
| <30, n = 154 | ≥30, n = 88 | ||
| BMI (kg/m 2 , mean and range) | 26 ± 3 (13.8–29.9) | 36 ± 6 (30.1–65) | <0.0001 |
| Age (yrs, mean ± SD) | 53 ± 17 | 51 ± 15 | 0.39 |
| Men | 64% | 72% | 0.21 |
| Race | |||
| Caucasian | 63% | 64% | 0.92 |
| African-American | 21% | 27% | 0.25 |
| Other | 16% | 9% | 0.12 |
| Family history of HC | 24% | 13% | 0.07 |
| Family history of sudden cardiac death | 14% | 12% | 0.72 |
| Family history of coronary artery disease | 22% | 18% | 0.53 |
| Diabetes mellitus | 8% | 15% | 0.08 |
| Dyslipidemia | 45% | 53% | 0.26 |
| Smoker | 21% | 31% | 0.17 |
| Hypertension | 37% | 55% | 0.008 |
| Systolic blood pressure at rest (mm Hg, mean ± SD) | 130 ± 19 | 134 ± 18 | 0.12 |
| Diastolic blood pressure at rest (mm Hg, mean ± SD) | 90 ± 28 | 88 ± 26 | 0.62 |
| Cardiovascular medications | |||
| β Blockers | 67% | 68% | 0.94 |
| Calcium antagonists | 25% | 34% | 0.16 |
| Disopyramide | 3% | 3% | 0.86 |
| ACE inhibitors | 16% | 33% | 0.004 |
| Diuretics | 15% | 22% | 0.17 |
| Clinical presentation | |||
| Chest pain | 18% | 38% | 0.002 |
| Dyspnea | 42% | 57% | 0.04 |
| New York Heart Association ≥II | 30% | 49% | 0.003 |
| Syncope | 11% | 13% | 0.62 |
| Paroxysmal atrial fibrillation | 10% | 8% | 0.59 |
| Persistent atrial fibrillation | 2% | 0% | 0.29 |
Differences in echocardiographic and exercise parameters between study groups are listed in Table 2 . Obese versus nonobese patients with HC did not significantly differ with respect to basal and midseptal wall thickness, but a significant difference in posterior wall thickness and LV mass index was observed ( Table 2 ). End-diastolic and end-systolic volumes and stroke volume index were also significantly greater in obese patients. No significant differences were found in parameters associated with LV remodeling, LV systolic and diastolic function; global longitudinal systolic strain was also similar between the 2 groups. There was no difference in LV outflow tract diameter and LV pressure gradients at rest ( Table 2 ). However, obese patients showed a trend toward higher stress-induced LV pressure gradients compared with nonobese patients. Nonobstructive, labile obstructive, and basal obstructive cases were equally distributed in the whole sample (36%, 37%, and 27%, respectively). When stratified by obesity, no differences were noted in basal obstruction, but the prevalence of labile obstruction appeared relatively greater in obese patients (46% vs 33%, p = 0.06). Upon excluding the 64 patients with obstructive physiology at rest (41 nonobese and 23 obese, with LV pressure gradients of 69 ± 36 vs 60 ± 33 mm Hg, p = 0.33, respectively), obese patients had significantly higher inducible LV pressure gradients (52 ± 53 vs 38 ± 34 mm Hg, p = 0.045, respectively; Figure 1 ) and a significantly greater chance of developing LV outflow tract obstruction during stress (62% vs 45%, p = 0.035) than those of their nonobese counterparts. Prevalence of SAM was similar in obese and nonobese patients and so were proportions of complete and incomplete SAM ( Table 2 ).
| Variable | BMI (kg/m 2 ) | p | |
|---|---|---|---|
| <30 (n = 154) | ≥30 (n = 88) | ||
| LV morphology | |||
| Septal wall thickness, basal segment (mm) | 18.6 ± 5.1 | 19.4 ± 4.4 | 0.21 |
| Septal wall thickness, mid segment (mm) | 18.7 ± 6.2 | 19.1 ± 6.5 | 0.62 |
| Maximal septal wall thickness (mm) | 20.5 ± 0.6 | 21.4 ± 0.5 | 0.22 |
| Posterior wall thickness (mm) | 10.9 ± 2.7 | 11.9 ± 3.1 | 0.01 |
| Septal-to-posterior wall thickness ratio | 1.95 ± 0.6 | 1.89 ± 0.6 | 0.46 |
| LV mass index (g/m 2.7 ) | 58 ± 22 | 67 ± 23 | 0.003 |
| LV end-diastolic volume index (ml/m 2.7 ) | 23.4 ± 7 | 26.2 ± 8 | 0.007 |
| LV end-systolic volume index (ml/m 2.7 ) | 8.1 ± 3.5 | 9.2 ± 3.6 | 0.02 |
| Stroke volume index (ml/m 2.7 ) | 15.3 ± 4.9 | 17 ± 6 | 0.03 |
| Relative wall thickness | 2 ± 0.6 | 2 ± 0.6 | 0.68 |
| LV function | |||
| E/A ratio | 1.32 ± 0.6 | 1.20 ± 0.4 | 0.13 |
| Septal mitral annulus early diastolic velocity (Em, cm/s) | 4.3 ± 1.7 | 4.2 ± 1.5 | 0.70 |
| E/Em ratio | 22.6 ± 12.6 | 22.3 ± 9.8 | 0.87 |
| Left atrial volume index (g/m 2 ) | 38.7 ± 21.7 | 36.5 ± 13.5 | 0.38 |
| LV ejection fraction (%) | 66 ± 9 | 65 ± 10 | 0.24 |
| Global longitudinal systolic strain (%) | −15 ± 3 | −15 ± 3 | 0.41 |
| LV outflow tract parameters | |||
| LV outflow tract diameter (cm) | 2.1 ± 0.3 | 2.2 ± 0.3 | 0.15 |
| LV outflow tract peak gradient at rest (mm Hg) | 27 ± 33 | 25 ± 28 | 0.72 |
| LV outflow tract peak gradient during Valsalva (mm Hg) | 30 ± 31 (n = 134) | 39 ± 40 (n = 83) | 0.05 |
| LV outflow tract peak gradient during treadmill (mm Hg) | 53 ± 43 (n = 120) | 66 ± 57 (n = 64) | 0.08 |
| LV outflow tract peak gradient during amyl nitrite inhalation (mm Hg) | 41 ± 41 (n = 106) | 53 ± 57 (n = 61) | 0.11 |
| Maximal inducible LV outflow tract peak gradient (mm Hg) | 55 ± 46 (n = 145) | 67 ± 57 (n = 86) | 0.09 |
| Dynamic LV outflow tract obstruction | |||
| Nonobstructive | 40% | 28% | 0.06 |
| Labile obstruction (stress-induced) | 33% | 46% | 0.06 |
| Basal obstruction | 27% | 26% | 0.93 |
| SAM of the mitral valve | |||
| No SAM | 18% | 13% | 0.30 |
| Incomplete SAM | 59% | 64% | 0.59 |
| Complete SAM | 23% | 23% | 0.95 |
| Treadmill exercise | n = 117 | n = 66 | |
| METs | 10.6 ± 4.2 | 9 ± 3.5 | 0.007 |
| Percentage exercise predicted heart rate | 86% | 84% | 0.65 |
| Systolic blood pressure at peak exercise (mm Hg) | 169 ± 39 | 173 ± 36 | 0.58 |
| Diastolic blood pressure at peak exercise (mm Hg) | 82 ± 22 | 80 ± 16 | 0.53 |
| Chest pain | 9% | 20% | 0.04 |
| Shortness of breath | 48% | 49% | 0.90 |
| Arrhythmia | 2% | 0% | 0.57 |
| Exercise time (seconds) for Bruce protocols | 603 ± 184 (n = 63) | 512 ± 212 (n = 34) | 0.03 |
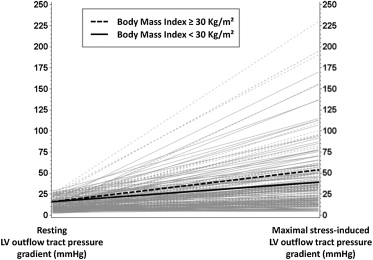
Treadmill exercise was performed by about 75% of patients in each group (117 of 154 nonobese and 66 of 88 obese). Functional capacity, expressed as METs, and exercise time (considered only for those who underwent the same Bruce protocol) were significantly lower in obese versus nonobese patients ( Table 2 ), and obese patients more frequently reported chest pain during the test. No differences were found for heart rate and blood pressure at peak exercise ( Table 2 ).
BMI showed a statistically significant negative association with METs, in both univariate and multivariate models ( Table 3 ). Hypertension and LV outflow tract obstruction severity were also responsible for significant reduction of exercise tolerance in our patients ( Table 3 ). When LV outflow tract obstruction severity was substituted with SAM severity or maximal inducible LV pressure gradients, results did not significantly change because of the high correlation between LV outflow tract obstruction severity and these 2 parameters (r s = 0.48, p <0.0001; r s = 0.85, p <0.0001, respectively). A significant interaction was found between BMI and hypertension in the model predicting METs (p = 0.04), and multiple comparisons between patients with and without obesity and hypertension revealed lesser METs in hypertensive obese patients than in others ( Figure 2 ).



