Previous studies have compared bivalirudin and unfractionated heparin (UFH) plus the routine use of glycoprotein IIb/IIIa inhibitors. They have demonstrated that bivalirudin can decrease bleeding complications without a significant increase in ischemic complications, resulting in a better net clinical outcome, as defined by the efficacy (ischemic complications) or safety (bleeding complications) end point. The aim of the present study was to compare bivalirudin and UFH plus protamine in patients undergoing elective percutaneous coronary intervention and pretreated with clopidogrel and aspirin. We randomly assigned 850 patients with stable or unstable coronary artery disease to bivalirudin or UFH followed by protamine at the end of the percutaneous coronary intervention. The primary end point was in-hospital major bleeding. The main secondary end points were the 1-month composite of death, myocardial infarction, unplanned target vessel revascularization; and the 1-month net clinical outcome. The rate of major bleeding (primary end point) was 0.5% in patients randomized to bivalirudin and 2.1% in patients randomized to UFH (p = 0.033). At 30 days, the rate of major bleeding was 0.9% in the bivalirudin arm and 2.8% in the UFH arm (p = 0.043). The composite of death, myocardial infarction, and target vessel revascularization rate and the net clinical outcome rate was 2.8% and 6.4% (p = 0.014) and 3.3% and 7.8% (p = 0.004), respectively, in the bivalirudin and UFH arms. In conclusion, in percutaneous coronary intervention patients pretreated with clopidogrel and aspirin, bivalirudin was associated with less major bleeding and fewer ischemic complications and a better net clinical outcome than UFH.
Compared to unfractionated heparin (UFH), bivalirudin has 2 major advantages in terms of bleeding risk, a highly predictable anticoagulant effect at a standardized dose and a short duration of the anticoagulant effect (drug plasma half-life 25 minutes). The latter can be canceled by the neutralization of heparin by protamine at the end of the procedure. The aim of the present randomized study was to determine whether bivalirudin remains superior to UFH plus protamine in patients undergoing percutaneous coronary intervention (PCI) who have been pretreated with aspirin and clopidogrel.
Methods
All patients undergoing PCI and pretreated with aspirin (325 mg) and a 600-mg loading dose of clopidogrel ≥6 hours before PCI were considered eligible for enrollment. The exclusion criteria were acute ST-segment elevation myocardial infarction; PCI for chronic total occlusion; renal insufficiency (creatinine clearance rate <30 ml/min or serum creatinine >3 mg/dl) or dependence on renal dialysis; co-morbid conditions with a life expectancy of <1 year; active bleeding, bleeding diathesis, or recent major surgery (<15 days); gastrointestinal or genitourinary bleeding within the previous 6 weeks; pretreatment with UFH or low-molecular-weight heparin or bivalirudin before PCI; uncontrolled hypertension >180/110 mm Hg unresponsive to therapy; relevant hematologic abnormalities (hemoglobin <10 g/dl or platelet count <100 × 10 9 /L); allergy to the study medications; a history of heparin-induced thrombocytopenia; and age <18 years. The institutional ethics committee approved the study, and all patients provided written informed consent. Allocation to treatment was done using a computer-generated sequence. The patients were assigned equally in an open-label fashion to UFH or bivalirudin, which were started immediately before PCI. UFH was administered as an intravenous bolus of 100 IU/kg body weight; additional boluses were administered to achieve an activated clotting time of 250 to 300 seconds during the entire procedure. At the end of the procedure, protamine sulfate was administered at a dose of 0.5 mg/100 IU of UFH used. Bivalirudin was administered as an intravenous bolus dose of 0.75 mg/kg, followed by infusion of 1.75 mg/kg/hour for the duration of the procedure. The use of glycoprotein IIb/IIIa inhibitors (GPIs) was at discretion of the operator, and abciximab was the only GPI allowed. All patients underwent femoral sheath removal immediately after PCI, and closure devices were used routinely. After PCI, dual antiplatelet treatment, including clopidogrel 75 mg/day and aspirin 325 mg/day was recommended for ≥1 year in all patients.
The primary end point of the present study was in-hospital major bleeding, defined according to the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events (REPLACE) 2 trial criteria. The diagnosis of major bleeding required the presence of intracranial, intraocular, or retroperitoneal hemorrhage, clinically overt blood loss resulting in a decrease in hemoglobin of >3 g/dl, any decrease in hemoglobin of >4 g/dl, or the transfusion of ≥2 units of packed red blood cells or whole blood. Other prespecified end points were (1) the 30-day composite of ischemia (death from any cause, myocardial infarction, unplanned revascularization for ischemia); (2) the 30-day net clinical outcome, defined as the 30-day composite ischemia or 30-day major bleeding; (3) 30-day minor bleeding; (4) 30-day entry site vascular complications; (5) 6-month mortality; (6) the 6-month composite of death and myocardial infarction; and (7) 6-month unplanned target vessel revascularization. Definite and probable stent thrombosis were defined according the Academic Research Consortium criteria. The myocardial infarction definition included the following criteria: electrocardiographic changes consistent with myocardial infarction or cardiac biomarker elevation (creatine kinase-MB or troponin I at one measurement 3 times greater than the upper normal limit) or cardiac biomarker re-elevation in patients with pre-PCI values greater than the upper normal limit (≥50% more than the previous nadir with documentation that the cardiac biomarker levels were decreasing before PCI). Minor bleeding was defined according to the REPLACE 2 criteria as clinical overt bleeding that did not meet the criteria for major bleeding. Entry site vascular complications included all complications requiring percutaneous or surgical intervention or blood transfusion. All events were adjudicated by an event adjudication committee whose members were unaware of the treatment assignments.
The study was designed on the basis of the superiority principle: the primary end point rate for patients allocated to the UFH group would be greater than for patients allocated to bivalirudin. We assumed that the primary end point would occur in 6% of the UFH group and in 2% of the bivalirudin group, for a 66% risk reduction with bivalirudin. The planned enrollment of 850 patients provided 91% power for detecting this reduction at an α level of 0.05. Categorical variables are expressed as frequencies and proportions and were compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous data are summarized as the mean ± SD and were compared using Student’s t test. The Kaplan-Meier method was used to estimate the 6-month event rates, and comparisons between the 2 treatment groups were performed using the log-rank statistic. Univariate and multivariate logistic regression analyses were performed to evaluate the independent contribution of the clinical, angiographic, and procedural variables to the 30-day end points. Variables with p <0.10 were entered into the multivariate model. The interaction terms for subgroup analysis (abciximab use vs no abciximab use) were tested in a logistic regression model. All analyses were performed in a blinded manner regarding the randomly assigned treatment and on an intention-to-treat basis. An α level of 0.05 was considered statistically significant. All tests were 2 sided. The analyses were performed using the Statistical Package for Social Sciences, version 11.5 (SPSS, Chicago, Illinois).
This trial was registered with Clinical Trials gov (number NCT00448461 ).
Results
From October 2006 to July 2008, 850 patients were enrolled in the present study ( Figure 1 ).
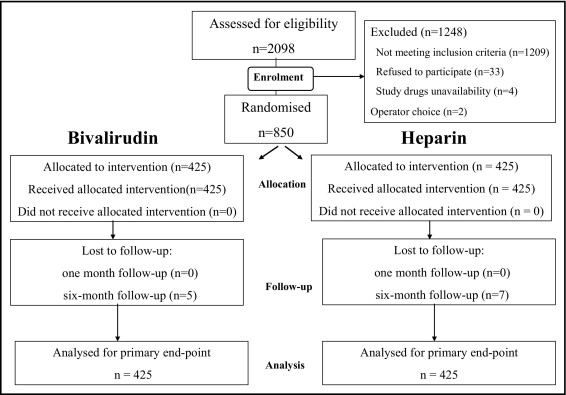
Table 1 lists the baseline characteristics. The 2 groups were well-matched in all baseline characteristics. Among patients with unstable angina, a Thrombolysis In Myocardial Infarction risk score of ≥5 was revealed in 19% of cases. Table 2 lists the procedural characteristics. Abciximab was used in 179 patients (21.1%), and its use was more frequent in the UFH arm than in the bivalirudin arm (27.6% vs 14.6%, p = 0.0001). The abciximab-treated patients more frequently had a greater risk profile than to the patients who did not receive the drug ( Table 3 ). Among the patients who received abciximab, no differences were found between those randomized to UFH or bivalirudin.
| Variable | Bivalirudin (n = 425) | Heparin (n = 425) | p Value |
|---|---|---|---|
| Age (years) | 68.7 ± 10.6 | 69.1 ± 10.6 | 0.544 |
| Men | 329 (77%) | 319 (75%) | 0.420 |
| Hypertension | 249 (59%) | 254 (61%) | 0.489 |
| Diabetes mellitus | 91 (21%) | 92 (22%) | 0.818 |
| Total cholesterol >200 mg/dl | 204 (48%) | 210 (51%) | 0.492 |
| Current smoker | 76 (18%) | 70 (17%) | 0.675 |
| Body mass index (kg/m 2 ) | 26.50 ± 3.07 | 26.13 ± 3.39 | 0.957 |
| Previous myocardial infarction | 172 (41%) | 158 (38%) | 0.443 |
| Myocardial infarction ≤30 days | 50 (12%) | 38 (9%) | 0.213 |
| Previous percutaneous coronary intervention | 234 (55%) | 233 (56%) | 0.780 |
| Previous coronary surgery | 42 (10%) | 32 (8%) | 0.267 |
| Serum creatinine (mg/dl) | 0.99 ± 0.62 | 1.02 ± 0.76 | 0.529 |
| Indication for procedure | |||
| Stable angina pectoris | 190 (45%) | 171 (40%) | 0.291 |
| Unstable angina pectoris | 117 (27%) | 112 (26%) | 0.844 |
| Positive stress result or other | 118 (28%) | 142 (33%) | 0.074 |
| Increased baseline troponin I | 62 (15%) | 79 (19%) | 0.109 |
| Left ventricular ejection fraction (%) | 48 ± 12 | 46 ± 12 | 0.125 |
| Multivessel coronary disease | 224 (53%) | 242 (58%) | 0.118 |
| Variable | Bivalirudin (n = 425) | Heparin (n = 425) | p Value |
|---|---|---|---|
| Rotational atherectomy | 7 (2%) | 10 (2%) | 0.435 |
| Intra-aortic balloon pump | 3 (1%) | 2 (1%) | 0.654 |
| Stenting | 360 (85%) | 373 (88%) | 0.196 |
| Bare-metal stent | 38 (9%) | 45 (11%) | 0.419 |
| Drug-eluting stent | 322 (76%) | 328 (77%) | 0.628 |
| Abciximab | 62 (15%) | 117 (28%) | 0.0001 |
| Activated clotting time (seconds) | 327 ± 36 | 297 ± 41 | 0.0001 |
| Fluoroscopic time (minutes) | 10.6 ± 10.3 | 10.7 ± 9.1 | 0.779 |
| Femoral arterial access | 418 (98%) | 415 (98%) | 0.462 |
| Femoral hemostasis | 0.516 | ||
| Closure device | 380 (91%) | 374 (91%) | |
| Manual compression | 45 (9%) | 51 (10%) | |
| Treated coronary vessels (n) | 595 | 589 | |
| Vessel location | 0.277 | ||
| Left main | 28 (5%) | 31 (5%) | |
| Left anterior descending | 259 (43%) | 257 (44%) | |
| Right | 134 (23%) | 148 (25%) | |
| Left circumflex | 156 (26%) | 141 (24%) | |
| Venous bypass graft | 18 (3%) | 12 (2%) |
| Variable | Abciximab | p Value | |
|---|---|---|---|
| No (n = 671) | Yes (n = 179) | ||
| Age (years) | 69 ± 12 | 69 ± 11 | 0.985 |
| Diabetes mellitus | 137 (21%) | 46 (26%) | 0.162 |
| Previous myocardial infarction | 281 (43%) | 48 (27%) | 0.0001 |
| Unstable angina pectoris | 149 (23%) | 80 (45%) | 0.0001 |
| Raised baseline troponin I | 88 (14%) | 53 (30%) | 0.0001 |
| Multivessel coronary disease | 349 (53%) | 116 (65%) | 0.004 |
| Left main intervention | 27 (4%) | 20 (11%) | 0.0001 |
Most patients in both arms had immediate successful entry site hemostasis using closure devices. No patient in the UFH arm experienced negative reactions, including no allergic reactions, after protamine administration.
The in-hospital bleeding and 30-day clinical outcomes are listed in Table 4 . The 1-month follow-up rate was 100%. The rate of major bleeding (primary end point) was 0.5% in the bivalirudin arm and 2.1% in the UFH arm (p = 0.033). In-hospital major bleeding events included 1 case of pericardial bleeding and 1 case of gastrointestinal bleeding in the bivalirudin arm and 1 case each of fatal rupture of an abdominal aortic aneurysm, retroperitoneal hematoma, and pericardial bleeding and 6 blood transfusions in the UFH arm. At 30 days, the major bleeding rate had increased to 0.9% in the bivalirudin arm and 2.8% in the UFH arm (p = 0.043). The difference between the 2 arms did not result from the more frequent use of abciximab in the UFH arm because among the patients who did not receive GPI treatment, the major bleeding rate was more than threefold greater in the UFH arm than in the bivalirudin arm (2.6% and 0.8%, respectively). No major bleeding occurred from the vascular access site after sheath removal. No patient with major bleeding required discontinuation of clopidogrel treatment. In both arms, 1/2 of the patients with major bleeding had had an adverse ischemic event at 30 days of follow-up.
| Variable | Bivalirudin (n = 425) | Heparin (n = 425) | p Value |
|---|---|---|---|
| Major bleeding | |||
| In-hospital | 2 (0.5%) | 9 (2.1%) | 0.033 |
| 1 Month | 4 (0.9%) | 12 (2.8%) | 0.043 |
| Composite end point ⁎ | 12 (2.8%) | 27 (6.4%) | 0.014 |
| Death | 1 (0.2%) | 6 (1.4%) | 0.069 † |
| Myocardial infarction | 11 (2.4%) | 20 (4.5%) | 0.098 |
| Q-wave | 1 | 1 | |
| Non–Q-wave | 10 | 19 | |
| Definite stent thrombosis | 2 (0.5%) | 1 (0.2%) | 0.563 |
| Acute | 1 | 0 | |
| Subacute | 1 | 1 | |
| Target vessel revascularization | 2 (0.4%) | 3 (0.7%) | 0.686 † |
| Net clinical outcome | 14 (3.3%) | 33 (7.8%) | 0.004 |
| Minor bleeding | 10 (2.4%) | 10 (2.4%) | 0.997 |
| Vascular access complications | 5 (1.2%) | 8 (1.9%) | 0.399 |
| Abciximab-treated patients (n = 179) | 62 | 117 | |
| Major bleeding | 1 (1.6%) | 4 (3.4%) | 0.660 † |
| Composite end point ⁎ | 4 (6.5%) | 7 (6.0%) | 0.901 |
| Net clinical outcome | 5 (8.1%) | 11 (9.4%) | 0.765 |
| Non–abciximab-treated patients (n = 671) | 363 | 308 | |
| Major bleeding | 3 (0.8%) | 8 (2.6%) | 0.072 |
| Composite end point ⁎ | 8 (2.2%) | 21 (6.8%) | 0.003 |
| Net clinical outcome | 9 (2.5%) | 23 (7.5%) | 0.003 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


