Implantable cardioverter–defibrillator (ICD) therapy decreases arrhythmic and all-cause mortality in patients at high risk of sudden death. However, its clinical benefit in elderly patients is uncertain. The aim of this study was to assess the long-term efficacy of ICD treatment in elderly patients and to identify markers of successful ICD therapy and risk factors of mortality. We performed multivariate analysis of a prospective long-term database from 2 tertiary care centers including 936 consecutive patients with an ICD. Predictors of ICD therapy and risk factors for mortality were assessed in patients ≥75 years old at ICD implantation compared to younger patients. Mean follow-up time was 43 ±40 months. Rates of ICD therapy were similar in the 2 age groups. No significant predictors of ICD therapy could be identified in older patients. Median estimated survival was 132 months in patients <75 years and 81 months in those ≥75 years old (p = 0.006). Decreased ejection fraction (hazard ratio 1.62 per 10% decrease, p = 0.03) and impaired renal function (hazard ratio 1.57 per 10 ml/kg/m 2 decrease in estimated glomerular filtration rate, p = 0.02) were independent risk factors of mortality in patients ≥75 years old. However, mortality of older patients was similar to that of the age-matched general population irrespective of delivery of ICD therapy. In conclusion, ICD therapy is effective for treatment of life-threatening arrhythmias in all age groups. However, prevention of sudden cardiac death may have limited impact on overall mortality in older patients. Despite a similar rate of appropriate ICD therapies, risk of death is increased 1.6-fold in ICD recipients ≥75 years old compared to younger patients. Patients with decreased ejection fraction and impaired renal function are at highest risk.
In the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II), which included 204 patients (17%) ≥75 years old and which demonstrated a 31% relative risk decrease of all-cause mortality for implantable cardioverter–defibrillator (ICD) therapy compared to antiarrhythmic medications, subgroup analysis indicated that ICD recipients >75 years showed a 46% relative risk decrease in mortality. In the Sudden Cardiac Death in Heart Failure Trial (SCD-HEFT), which included 1,551 patients (46%) ≥65 years old, ICD implantation was the superior primary prevention strategy compared to amiodarone in patients >65 years old, although a greater mortality decrease was demonstrated in patients <60 years. However, no randomized controlled studies have investigated the role of ICD specifically in older adults. Furthermore, analysis of pooled results of secondary prevention ICD trials and primary prevention trials suggests that elderly patients may derive less benefit from an ICD than younger patients. ICD prescription in older patients requires particular considerations because of common co-morbidities and higher rates of noncardiac mortality. The present study compared the long-term efficacy of ICD treatment in patients ≥75 years old to those <75 years old.
Methods
In prospective registries from 2 university hospitals in Zurich, Switzerland and Innsbruck, Austria, we acquired data from 936 patients undergoing initial ICD implantation from 1987 through 2009. These hospitals serve as tertiary referral centers and provide community health care services. Men and women who were >18 years of age were eligible for the study if they had a signed informed consent on file at the implantation center before ICD implantation and had ≥1 of the following situations: survival of cardiac arrest with loss of consciousness owing to ventricular tachyarrhythmia, recurrent hemodynamically poorly tolerated ventricular tachycardia (VT), previous myocardial infarction and left ventricular ejection fraction ≤35%, and (before publication of results of the MADIT-II) a documented episode of nonsustained VT and inducible ventricular tachyarrhythmia. All patients attended regular follow-up visits, which were scheduled at 3- to 6-month intervals. Device programming was individualized according to patients’ underlying cardiac disease and tachycardia characteristics.
Patient demographics, ventricular function, revascularization, arrhythmia history, and clinical co-morbidities were assessed before device implantation. Clinical parameters and device-related parameters including occurrence of ventricular arrhythmias and respective ICD treatments were collected at time of implantation and during follow-up visits. Electrical storm was defined as 3 appropriate VT detections in 24 hours treated by antitachycardia pacing, shock, or eventually untreated but sustained in a VT monitoring zone. Mortality data were collected from hospital charts, primary care physicians, and patients’ families.
Descriptive statistics were performed for analysis of baseline variables. Categorical data are presented as frequency (percentage), whereas continuous variables are presented as mean ± SD. Baseline characteristics between age groups were compared using unpaired t test for continuous variables or Mann–Whitney test for non-normally distributed variables. Chi-square or Fisher’s exact test was used to compare categorical variables. Unadjusted survival curves were constructed using Kaplan–Meier estimates and differences in event-free survival were assessed with log-rank test. To appreciate the impact of age on mortality, survival curves presented with the expected survival of the age- and gender-matched general population were computed using the R Package Survival by Terry Therneau and the original R Port by Thomas Lumley (2009; Survival analysis including penalized likelihood, R 2.35-8, available at: http://CRAN.R-project.org/package=survival ). No health-related data were available from the general population control group.
Univariate Cox regression analysis was used to determine hazard ratios (HRs) for various prespecified clinical factors including age, gender, left ventricular function, electrocardiographic characteristics, underlying cardiac disease, family history of cardiac death, arterial hypertension, diabetes mellitus, and renal function. A multivariate Cox regression model was used to identify independent predictors of mortality and of appropriate device therapy. Forward stepwise regression was performed with a p value of 0.5 for entry and a p value of 0.1 for removal from the model. Assumption of proportionality was assessed with plots of the log–log (survival) versus log (survival time). For all analyses the null hypothesis was evaluated at a 2-sided significance level of 0.05. Statistical analysis was performed with SPSS 18 for Mac OSX (SPSS, Inc., Chicago, Illinois).
The study was conducted according to institutional and regional ethics guidelines.
The authors designed the study, collected and analyzed the data, and drafted and revised the report. All authors have read and agreed to the report as written.
Results
The study cohort was comprised of 936 patients (85% men, median age 62.7 years, interquartile range 55.3 to 69.3). Mean follow-up time was 43 ± 40 months. Rates of primary and secondary prevention indications for ICD implantation, left ventricular ejection fraction, New York Heart Association functional class, and QRS width were similar between patients <75 years old and those ≥75 years old; however, a history of syncope, atrial fibrillation, and impaired renal function was noted significantly more frequently in older patients ( Table 1 ).
| Variable | Age (years) | p Value | |
|---|---|---|---|
| <75 (n = 863) | ≥75 (n = 73) | ||
| Men | 727 (84%) | 64 (88%) | 0.50 |
| Age, median (interquartile range) | 62 (54–72) | 77 (76–79) | |
| Indication | |||
| Primary prevention | 366 (43%) | 23 (32)% | 0.08 |
| Secondary prevention | 495 (57%) | 50 (68%) | |
| Syncope | 166 (19%) | 28 (38%) | <0.01 |
| Cause | |||
| Coronary disease | 501 (58%) | 40 (55%) | 0.34 |
| Nonischemic dilated cardiomyopathy | 362 (42%) | 33 (45%) | |
| Left ventricular ejection fraction (%) | 30 ± 12 | 32 ± 12 | 0.14 |
| Left ventricular ejection fraction <35% | 609 (71%) | 37 (52%) | 0.001 |
| New York Heart Association functional class >II | 335 (39%) | 25 (35%) | 0.29 |
| Atrial fibrillation | 309 (36%) | 37 (51%) | 0.01 |
| QRS complex >120 ms | 444 (55%) | 43 (64%) | 0.09 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 69 ± 43 | 54 ± 19 | <0.01 |
| Device | |||
| 1-Chamber implantable cardioverter–defibrillator | 456 (53%) | 22 (30%) | |
| 2-Chamber implantable cardioverter–defibrillator | 187 (22%) | 31 (43%) | <0.01 |
| Cardiac resynchronization therapy—defibrillator | 119 (25%) | 20 (27%) | |
Changes in each patient’s medical treatment regimen over time precluded quantitative analysis. However, 91% of all patients were treated with β blockers at times during the study period, 44% with amiodarone, and 85% with angiotensin-converting enzyme inhibitors and/or angiotensin receptor antagonists, with no statistically significant difference between age groups.
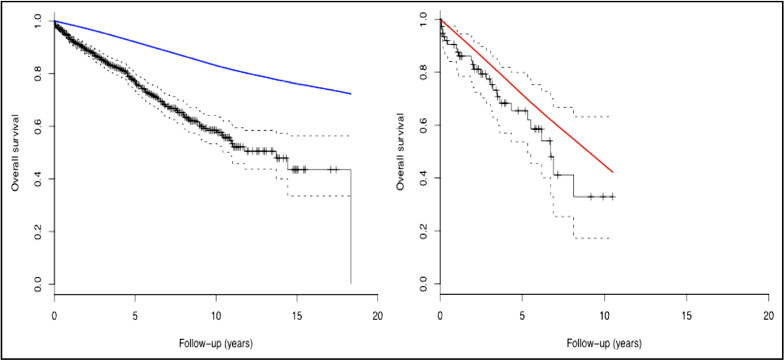
There were 214 deaths in the overall cohort; mortality rates were 22% in patients <75 years and 34% in patients ≥75 years old (p = 0.01). Median estimated survival was 132 months (95% confidence interval [CI] 99 to 164) in patients <75 years old and 81 months (95% CI 58 to 103) in those ≥75 years old (p = 0.006).
Figure 1 demonstrates survival curves of ICD patients combined with those of the age- and gender-matched general population. Survival of patients <75 years was decreased compared to the general population. In patients ≥75 years old the survival curve of the overall population was within the 95% CI of the estimated survival of ICD recipients.
ICD therapy was delivered in 42% of all patients. Thirty-four percent were treated with ≥1 episode of antitachycardia pacing and 33% of all patients received appropriate ICD shocks. Estimated median time to first appropriate ICD therapy was 57 months (95% CI 50 to 64). Kaplan–Meier curves ( Figure 2 ) demonstrated no significant difference in appropriate ICD therapies between the 2 age groups (p = 0.60).
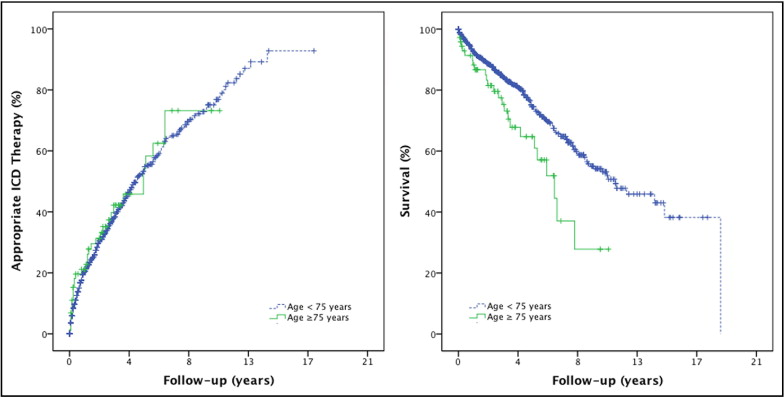
Twenty-one percent of all patients had inappropriate ICD shocks and electrical storms occurred in 5% of all patients. Lead revisions had to be performed in 19% of all ICD recipients. Ten percent of implanted devices had to be replaced because of premature battery depletion, and in 0.3% the ICD was explanted according to a patient’s request. Inappropriate ICD therapy occurred more often in patients <75 years old (22% vs 12% in patients ≥75 years old, p = 0.04). There was no difference in rates of appropriate therapies or adverse events between the 2 age groups. Univariate predictors of appropriate ICD therapy according to age groups are presented in Table 2 . Wide QRS complex and decreased ejection fraction were associated with a higher probability of appropriate ICD therapy in all patients, whereas male gender, presence of atrial fibrillation, a secondary prevention indication for ICD implantation, and impaired renal function were associated with delivery of ICD therapies only in younger patients. In multivariate analysis a QRS complex >120 ms, male gender, and impaired renal function were predictors of appropriate ICD therapy in patients <75 years. In older patients a wide QRS complex was the strongest independent predictor of ICD therapy, although reaching only borderline statistical significance ( Table 3 ).
| Variable | Age <75 Years (n = 863) | Age ≥75 Years (n = 73) | ||
|---|---|---|---|---|
| HR | p Value | HR | p Value | |
| Male gender | 1.66 | 0.003 | 0.79 | 0.66 |
| Age (per 10 years) | 1.08 | 0.08 | 0.71 | 0.64 |
| Secondary prevention indication | 1.44 | 0.003 | 1.35 | 0.44 |
| Coronary disease | 0.99 | 0.93 | 1.33 | 0.43 |
| Device | ||||
| 2-Chamber implantable cardioverter–defibrillator | 1.25 | 0.08 | 1.16 | 0.75 |
| Cardiac resynchronization therapy—defibrillator | 1.04 | 0.78 | 1.75 | 0.23 |
| Left ventricular ejection fraction (by 10% decrease) | 1.1 | 0.03 | 1.35 | 0.05 |
| Left ventricular ejection fraction <35% | 1.30 | 0.03 | 2.19 | 0.04 |
| New York Heart Association functional class >II | 1.22 | 0.07 | 1.55 | 0.25 |
| Atrial fibrillation | 1.34 | 0.006 | 1.06 | 0.88 |
| QRS complex >120 ms | 1.48 | <0.01 | 2.32 | 0.06 |
| Estimated glomerular filtration rate (by 10-ml/kg/m 2 decrease) | 1.13 | <0.001 | 1.0 | 1.0 |
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age <75 years (n = 863) | |||
| QRS complex >120 ms | 1.44 | 1.15–1.81 | 0.002 |
| Male gender | 1.86 | 1.31–2.65 | <0.001 |
| Estimated glomerular filtration rate (per10-ml/kg/m 2 decrease) | 1.10 | 1.03–1.18 | 0.007 |
| Age ≥75 years (n = 73) | |||
| QRS complex >120 ms | 1.84 | 0.99–4.86 | 0.053 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


