There are limited safety and effectiveness data comparing glycoprotein IIb/IIIa inhibitors in the setting of primary percutaneous coronary intervention. In this substudy of the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, the clinical and bleeding outcomes of eptifibatide versus abciximab were evaluated in patients with ST-segment elevation myocardial infarction who underwent percutaneous coronary intervention. Three-year clinical outcomes of patients in the heparin plus glycoprotein IIb/IIIa inhibitor arm were compared according to treatment with abciximab (n = 907) versus eptifibatide (n = 803). Adjudicated end points included major adverse cardiovascular events (MACEs; mortality, reinfarction, ischemia-driven target vessel revascularization, or stroke), major bleeding, and net adverse clinical events (MACEs or major bleeding). Propensity score matching was used to identify 1,342 matched cases (671 each in the abciximab and eptifibatide groups). Multivariate analysis was performed in the entire cohort and the propensity-matched groups. At 3-year follow-up, eptifibatide and abciximab resulted in nonsignificantly different rates of MACEs (18.3% vs 19.6%, hazard ratio [HR] 0.93, 95% confidence interval [CI] 0.74 to 1.16, p = 0.51), major bleeding (10.7% vs 11.9%, HR 0.90, 95% CI 0.67 to 1.19, p = 0.44), and net adverse clinical events (24.5% vs 25.5%, HR 0.96, 95% CI 0.79 to 1.17, p = 0.69). Similarly, at 3 years by multivariate analysis, there was no statistically significant difference between abciximab and eptifibatide for net adverse clinical events (HR 0.89, 95% CI 0.73 to 1.09, p = 0.27), MACEs (HR 0.96, 95% CI 0.77 to 1.20, p = 0.73), and major bleeding (HR 1.05, 95% CI 0.78 to 1.41, p = 0.75). The propensity-matched groups also had similar outcomes. In conclusion, abciximab and eptifibatide have comparable bleeding risks and clinical efficacy in primary percutaneous coronary intervention.
In the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, 3,602 patients with ST-segment elevation myocardial infarctions were preloaded with a thienopyridine and randomized to receive heparin plus glycoprotein IIb/IIIa inhibitors (GPIs) or bivalirudin during primary percutaneous coronary intervention (PCI). In the present analysis, we examined the early and late outcomes of those patients assigned to heparin plus GPIs according to treatment with abciximab or eptifibatide.
Methods
The HORIZONS-AMI trial design has been described previously. In brief, this study was a large-scale, prospective, 2 × 2 factorial randomized trial of 3,602 patients enrolled at 123 centers worldwide. The study protocol was approved by the institutional review board or ethics committee at each center, and written informed consent was obtained from all patients. Patients were randomized 1:1 in the emergency room to anticoagulation with unfractionated heparin plus routine use of a GPI (control arm) or bivalirudin plus provisional GPI use reserved for refractory thrombotic complications. After angiography, patients with lesions eligible for stenting underwent a second randomization (3:1) to either Taxus Express-2 paclitaxel-eluting stents or otherwise identical uncoated Express-2 bare-metal stents (Boston Scientific Corporation, Natick, Massachusetts).
The choice of GPI was made on an institutional level, and each center declared before study initiation if its study patients would be treated with abciximab or eptifibatide. Randomization was then stratified by the choice of GPI. Abciximab dose was a 0.25 mg/kg bolus followed by a 12-hour infusion at 0.125 μg/kg/min (maximum 10 μg/min). Eptifibatide dosing included 2 boluses at 180 μg/kg administered 10 minutes apart and followed by an infusion at 2.0 μg/kg/min for 12 to 18 hours. Doses were adjusted for renal impairment per the United States Food and Drug Administration label. Unfractionated heparin was administered intravenously as a 60 IU/kg bolus with subsequent boluses to achieve an activated clotting time of 200 to 250 seconds. Aspirin (100 to 324 mg orally or 500 mg intravenously) was administered daily during the index hospitalization, and a minimum of 75 mg/day was prescribed indefinitely after discharge. The initial loading dose of thienopyridine was left to the investigator’s discretion, although a ≥300-mg clopidogrel loading dose was required to be administered in the emergency room. Randomization was also stratified by thienopyridine loading dose (300 vs 600 mg). Clopidogrel (75 mg/day) was mandated for ≥6 months (≥1 year recommended) after discharge. Ticlopidine was permitted in the case of clopidogrel allergy or unavailability.
The designated primary end points of HORIZONS-AMI have previously been described. The composite end points used in this study included (1) major adverse cardiovascular events (MACEs), a composite of all-cause death, reinfarction, ischemia-driven target vessel revascularization, or stroke, and (2) net adverse clinical events (NACEs), composed of MACEs or major bleeding not related to coronary artery bypass grafting. Major bleeding was defined as intracranial or intraocular bleeding, bleeding at the access site with a hematoma of >5 cm or requiring intervention, decrease in hemoglobin by 3 g/dl with a recognized bleeding source or 4 g/dl without an overt source, reoperation for bleeding, or requiring a blood transfusion. MACE and major bleeding end points were adjudicated by an independent clinical events committee (Cardiovascular Research Foundation, New York, New York) blinded to treatment assignment after review of source documents. Bleeding was also adjudicated according to the Thrombolysis In Myocardial Infarction (TIMI) and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) bleeding scales. Angiographic measures of baseline and postprocedural TIMI flow were determined at an independent core angiographic laboratory (Cardiovascular Research Foundation) as previously described. Baseline and 60-minute post-PCI electrocardiograms were interpreted at an independent electrocardiographic core laboratory (Cardiovascular Research Foundation). ST-segment deviation was evaluated using standardized techniques. ST-segment resolution from baseline to the 60-minute post-PCI electrocardiogram was categorized as complete (>70%), partial (30% to 70%), or absent (<30%).
Categorical variables were compared using the chi-square test. Continuous variables were displayed as median values with interquartile ranges and analyzed using Wilcoxon’s rank-sum test. Time-to-event outcomes using the Kaplan-Meier method were assessed with the log-rank test. The independent predictors of MACEs and NACEs at 30 days and 3 years and major bleeding at 30 days were identified using Cox proportional-hazards regression using a stepwise multivariate algorithm with entry and exit criteria set at p = 0.10. Variables included were limited to a maximum of 1 covariate per every 10 measured clinical events to avoid model overfitting. Candidate variables for multivariate analysis included age, gender, diabetes, hypertension, hyperlipidemia, history of heart failure, current cigarette smoking, history of myocardial infarction, history of PCI, history of coronary artery bypass grafting, creatinine clearance, anemia, platelet count, the left ventricular ejection fraction, Killip class, clopidogrel loading dose (600 mg vs other), symptom-to-balloon time, access site (radial vs femoral), baseline TIMI flow, the use of prerandomization heparin, and index PCI vessel. GPI use was forced into the multivariate model as an entry criteria.
To further account for confounding variables and bias, propensity matching was performed on the study cohort. A logistic regression model was fit for GPI use (abciximab vs eptifibatide) to pretreatment patient characteristics. Propensity score matching was performed using the Caliper matching algorithm and the SAS “GREEDY” macro (SAS Institute Inc., Cary, North Carolina). With GPI use included as an entry criteria, multivariate analysis was then performed on the propensity-matched population. Statistical analysis was performed using SAS versions 9.1.3 and 9.2.
Results
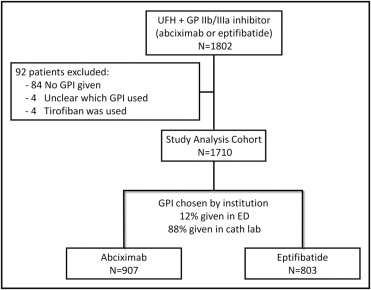
A total of 1,802 patients were assigned to receive unfractionated heparin plus a GPI. As shown in Figure 1 , 92 patients were excluded because GPIs were not given (n = 84), tirofiban was used (n = 4), or it was unclear which GPI was administered (n = 4). The present analysis cohort thus includes 1,710 patients, 907 (53.0%) treated with abciximab and 803 (47.0%) treated with eptifibatide. The baseline patient and angiographic characteristics of the groups are listed in Table 1 . Compared to patients treated with eptifibatide, those treated with abciximab were more likely female, with Killip class III or IV heart failure on presentation, with higher baseline rates of TIMI grade 0 or 1 flow, with lower baseline platelet counts, and with longer symptom-to-balloon times. Conversely, patients treated with eptifibatide had higher rates of previous PCI and coronary artery bypass grafting and use of femoral vascular access and closure device use.
| Variable | Abciximab | Eptifibatide | p Value |
|---|---|---|---|
| (n = 907) | (n = 803) | ||
| Age (years) | 61.3 (53.4–70.2) | 59.7 (52.5–69.9) | 0.14 |
| Men | 664 (73.2%) | 647 (80.6%) | 0.0003 |
| Diabetes mellitus | 148 (16.3%) | 149 (18.6%) | 0.22 |
| Hypertension ⁎ | 482 (53.1%) | 453 (56.4%) | 0.18 |
| Hyperlipidemia † | 379 (41.8%) | 351 (43.7%) | 0.42 |
| Current smoker | 551 (61.2%) | 503 (62.6%) | 0.28 |
| Body mass index (kg/m 2 ) | 27.1 (24.7–30.1) | 27.0 (24.6–30.1) | 0.95 |
| Previous myocardial infarction | 110 (12.1%) | 83 (10.3%) | 0.24 |
| Previous PCI | 85 (9.4%) | 102 (12.7%) | 0.02 |
| Previous coronary artery bypass grafting | 15 (1.7%) | 26 (3.2%) | 0.03 |
| Previous congestive heart failure | 30 (3.3%) | 23 (2.9%) | 0.60 |
| Left ventricular ejection fraction (%) | 50 (41–55) | 50 (42–60) | 0.0002 |
| Chronic kidney disease | 26 (2.9%) | 22 (2.7%) | 0.87 |
| Creatinine clearance (ml/min) ‡ | 86.8 (67.8–112.3) | 90.5 (69.1–111.5) | 0.29 |
| Baseline anemia § | 95 (11.6%) | 77 (9.7%) | 0.22 |
| Platelet count (×10 3 ) | 237 (201–286) | 251 (211–294) | 0.0003 |
| Platelet count <150 (×10 3 ) | 54 (5.9%) | 23 (2.9%) | 0.0018 |
| Killip class II–IV | 92 (10.1%) | 52 (6.5%) | 0.007 |
| Catheterization access | |||
| Femoral | 813 (89.6%) | 788 (98.1%) | <0.001 |
| Radial | 92 (10.1%) | 13 (1.6%) | <0.001 |
| Brachial | 2 (0.2%) | 2 (0.2%) | 1.0 |
| Primary management strategy | |||
| Primary PCI | 872 (96.1%) | 763 (95.0%) | 0.26 |
| Deferred PCI | 0 (0.0%) | 0 (0.0%) | 0 |
| Coronary artery bypass grafting without PCI | 8 (0.9%) | 12 (1.5%) | 0.24 |
| Medical management | 27 (3.0%) | 28 (3.5%) | 0.55 |
| Symptom-to-balloon time (minutes) | 233 (166–345) | 210 (154–322) | 0.006 |
| Index vessel for PCI | |||
| Left anterior descending | 378/940 (40.2%) | 358/819 (43.7%) | 0.14 |
| Left circumflex | 146/940 (15.5%) | 124/819 (15.1%) | 0.82 |
| Right coronary | 405/940 (43.1%) | 324/819 (39.6%) | 0.13 |
| Left main | 5/940 (0.5%) | 2/819 (0.2%) | 0.46 |
| Saphenous venous graft | 6/940 (0.6%) | 11/819 (1.3%) | 0.13 |
| Baseline TIMI flow grade | |||
| 0/1 | 626/939 (66.7%) | 502/815 (61.6%) | 0.03 |
| 2 | 54/939 (5.8%) | 51/819 (6.2%) | 0.67 |
| 3 | 864/939 (92.0%) | 748/819 (91.3%) | 0.61 |
| Number of stents implanted | 1.5 ± 0.8 | 1.6 ± 0.9 | 0.01 |
| Total length of stents (mm) | 24.0 (20.0–32.0) | 24.0 (18.0–36.0) | 0.22 |
| Stent implantation | |||
| Paclitaxel-eluting stent | 591/817 (72.3%) | 521/718 (72.6%) | 0.92 |
| Bare-metal stent | 236/817 (28.9%) | 207/718 (28.8%) | 0.98 |
| Closure device used | 189/782 (24.2%) | 259/751 (34.5%) | <0.0001 |
⁎ Defined as per patient report or medical chart review documentation of history of systemic arterial hypertension or current diagnosis.
† Defined as per patient report or medical chart review documentation of history of hyperlipidemia or current diagnosis.
‡ Creatinine clearance was calculated using the Cockcroft-Gault formula.
§ Baseline anemia was defined as hematocrit <39% for men and <36% for women.
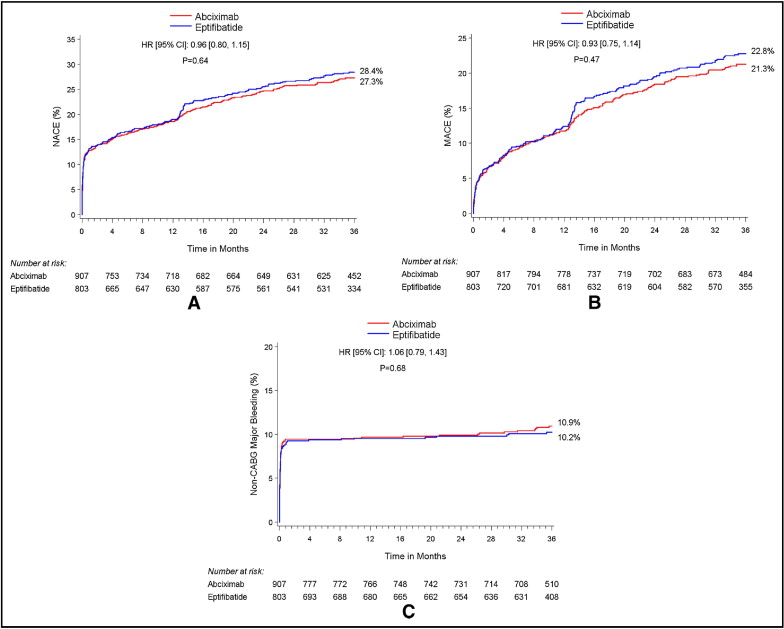
Immediate procedural revascularization success was high in the 2 groups, with TIMI grade 3 flow in the culprit vessel achieved in 92% of the abciximab group and 91% of the eptifibatide group (p = 0.60). The abciximab and eptifibatide treatment arms achieved similar rates of complete ST-segment resolution at 60 minutes ( Table 2 ). Rates of postprocedural thrombocytopenia (platelet count <100,000 cells/mm 3 ), were 5.3% with abciximab versus 2.1% with eptifibatide (p = 0.0008). Unadjusted safety and efficacy outcomes according to GPI treatment were not significantly different at 30 days and 3 years ( Table 3 , Figure 2 ). There were no significant differences in patient outcomes between the GPI groups when stratified by clopidogrel loading dose ( Table 4 ).
| Variable | Abciximab | Eptifibatide | p value |
|---|---|---|---|
| (n = 907) | (n = 803) | ||
| Baseline | |||
| Sum ST-segment deviation (mm) | 7.7 (4.5–13.2) | 7.5 (4.4–12.2) | 0.46 |
| ST-segment elevation | 787/810 (97.2%) | 719/748 (96.1%) | 0.26 |
| Location of ST-segment elevation ⁎ | n = 787 | n = 719 | |
| Anterior | 127 (16.1%) | 125 (17.4%) | 0.52 |
| Septal | 0 (0.0%) | 1 (0.1%) | 0.48 |
| Anteroseptal | 84 (10.7%) | 110 (15.3%) | 0.007 |
| Lateral | 23 (2.9%) | 18 (2.5%) | 0.62 |
| Inferior | 343 (43.6%) | 279 (38.8%) | 0.06 |
| Posterior | 2 (0.3%) | 1 (0.1%) | 1.00 |
| Inferoposterior | 4 (0.5%) | 11 (1.5%) | 0.05 |
| Inferolateral | 80 (10.2%) | 74 (10.3%) | 0.94 |
| Anterolateral | 108 (13.7%) | 90 (12.5%) | 0.49 |
| Inferoposterolateral | 16 (2.0% | 10 (1.4%) | 0.34 |
| 60 minutes after PCI | |||
| Sum ST-segment deviation (mm) | 2.0 (0.0–5.3) | 2.1 (0.5–5.2) | 0.52 |
| ST-segment resolution | n = 696 | n = 651 | |
| Complete (>70%) | 357 (51.3%) | 317 (48.7%) | 0.34 |
| Partial (30%–70%) | 191 (27.4%) | 194 (29.8%) | 0.34 |
| None (<30%) | 148 (21.3%) | 140 (21.5%) | 0.91 |
| Left bundle branch block | 10/778 (1.3%) | 9/731 (1.2%) | 0.93 |
| Q waves after PCI | 489/778 (62.9%) | 445/732 (60.8%) | 0.41 |
| Variable | 30-Day Outcomes | 3-Year Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Abciximab (n = 907) | Eptifibatide (n = 803) | HR (95% CI) | p Value | Abciximab (n = 907) | Eptifibatide (n = 803) | HR (95% CI) | p Value | |
| NACEs ⁎ | 12.7% (115) | 12.8% (103) | 0.99 (0.76–1.30) | 0.97 | 27.3% (242) | 28.4% (222) | 0.96 (0.80–1.15) | 0.64 |
| MACEs † | 5.3% (48) | 5.6% (45) | 0.95 (0.63–1.42) | 0.79 | 21.3% (187) | 22.8% (176) | 0.93 (0.75–1.14) | 0.47 |
| Death, all-cause | 3.2% (29) | 3.0% (24) | 1.07 (0.63–1.85) | 0.79 | 8.4% (74) | 7.0% (54) | 1.21 (0.85–1.72) | 0.28 |
| Cardiac | 3.0% (27) | 2.9% (23) | 1.04 (0.60–1.82) | 0.88 | 5.6% (49) | 4.5% (35) | 1.24 (0.80–1.91) | 0.33 |
| Noncardiac | 0.2% (2) | 0.1% (1) | 1.78 (0.16–19.59) | 0.63 | 3.0% (25) | 2.6% (19) | 1.16 (0.64–2.10) | 0.63 |
| Reinfarction | 1.3% (12) | 2.3% (18) | 0.59 (0.28–1.23) | 0.15 | 7.2% (60) | 9.3% (69) | 0.76 (0.54–1.08) | 0.12 |
| Q-wave | 1.0% (9) | 1.5% (12) | 0.67 (0.28–1.58) | 0.35 | 3.2% (27) | 4.7% (35) | 0.68 (0.41–1.12) | 0.13 |
| Non-Q-wave | 0.3% (3) | 1.0% (8) | 0.33 (0.09–1.26) | 0.09 | 4.1% (34) | 5.6% (41) | 0.73 (0.46–1.15) | 0.17 |
| Target vessel revascularization | 1.7% (15) | 2.1% (17) | 0.78 (0.39–1.57) | 0.49 | 11.6% (97) | 15.0% (111) | 0.76 (0.58–1.00) | 0.048 |
| Stent thrombosis | 0.2% (2) | 0.3% (2) | 0.88 (0.12–6.21) | 0.89 | 6.5% (50) | 6.4% (45) | 0.98 (0.65–1.46) | 0.92 |
| ARC definite | 0.1% (1) | 0.3% (2) | 0.44 (0.04–4.83) | 0.49 | 4.1% (32) | 4.8% (33) | 0.85 (0.53–1.39) | 0.53 |
| ARC probable | 0.1% (1) | 0.0% (0) | NA | 0.35 | 1.0% (8) | 1.0% (7) | 1.01 (0.36–2.77) | 0.99 |
| Stroke | 0.7% (6) | 0.5% (4) | 1.33 (0.38–4.72) | 0.65 | 2.3% (19) | 1.6% (12) | 1.41 (0.68–2.90) | 0.35 |
| Protocol major bleeding, non–coronary artery bypass grafting | 9.3% (84) | 8.9% (71) | 1.05 (0.77–1.44) | 0.74 | 10.9% (97) | 10.2% (81) | 1.06 (0.79–1.43) | 0.68 |
| Protocol major bleeding, all | 9.9% (89) | 11.4% (91) | 0.87 (0.65–1.16) | 0.34 | 11.7% (104) | 12.6% (100) | 0.92 (0.70–1.21) | 0.54 |
| Blood transfusion | 4.4% (40) | 3.3% (26) | 1.37 (0.84–2.25) | 0.21 | 5.7% (50) | 4.5% (35) | 1.27 (0.82–1.96) | 0.28 |
| TIMI major bleeding | 3.7% (33) | 5.4% (43) | 0.68 (0.43–1.07) | 0.09 | 4.5% (40) | 6.2% (49) | 0.72 (0.47–1.09) | 0.12 |
| TIMI minor bleeding | 4.7% (42) | 4.8% (38) | 0.98 (0.63–1.53) | 0.94 | 4.9% (44) | 5.2% (51) | 0.95 (0.62–1.46) | 0.83 |
| TIMI major or minor bleeding | 8.3% (75) | 10.0% (80) | 0.83 (0.61–1.14) | 0.24 | 9.3% (83) | 11.1% (88) | 0.83 (0.62–1.12) | 0.23 |
| GUSTO life-threatening or severe bleeding | 0.7% (6) | 0.5% (4) | 1.33 (0.38–4.71) | 0.66 | 0.9% (8) | 0.8% (6) | 1.18 (0.41–5.40) | 0.76 |
| GUSTO moderate bleeding | 4.3% (39) | 5.0% (40) | 0.87 (0.56–1.35) | 0.53 | 5.5% (48) | 6.0% (47) | 0.90 (0.60–1.35) | 0.62 |
⁎ Any MACEs or major bleeding events, excluding those related to coronary artery bypass grafting.
† Death, reinfarction, ischemic target vessel revascularization, or stroke.
| Variable | Abciximab + Clopidogrel 300 mg | Eptifibatide + Clopidogrel 300 mg | HR (95% CI) | p Value for Interaction | Abciximab + Clopidogrel 600 mg | Eptifibatide + Clopidogrel 600 mg | HR (95% CI) | p Value for Interaction |
|---|---|---|---|---|---|---|---|---|
| (n = 346) | (n = 276) | (n = 612) | (n = 565) | |||||
| NACEs ⁎ | 28.3% (96) | 33.4% (89) | 0.83 (0.63–1.11) | 0.22 | 26.1% (155) | 26.1% (142) | 1.01 (0.81–1.27) | 0.92 |
| MACEs † | 22.2% (75) | 25.4% (67) | 0.88 (0.63–1.23) | 0.45 | 20.1% (118) | 21.6% (116) | 0.92 (0.61–1.19) | 0.52 |
| Death | 8.0% (27) | 7.6% (20) | 1.08 (0.61–1.93) | 0.79 | 8.7% (51) | 6.5% (35) | 1.34 (0.87–2.07) | 0.18 |
| Cardiac | 5.9% (20) | 4.5% (12) | 1.34 (0.65–2.73) | 0.43 | 5.5% (32) | 4.2% (23) | 1.29 (0.75–2.20) | 0.35 |
| Noncardiac | 2.2% (7) | 3.2% (8) | 0.70 (0.25–1.93) | 0.49 | 3.4% (19) | 2.4% (12) | 1.45 (0.70–2.99) | 0.31 |
| Reinfarction | 6.6% (21) | 11.6% (30) | 0.55 (0.32–0.96) | 0.03 | 7.3% (41) | 8.3% (43) | 0.87 (0.57–1.33) | 0.52 |
| Q-wave | 2.8% (9) | 5.8% (15) | 0.48 (0.21–1.09) | 0.07 | 3.2% (18) | 4.0% (21) | 0.78 (0.42–1.47) | 0.45 |
| Non-Q wave | 3.8% (12) | 6.3% (16) | 0.59 (0.28–1.25) | 0.16 | 4.3% (24) | 5.5% (28) | 0.78 (0.45–1.35) | 0.38 |
| Death or reinfarction | 13.7% (46) | 16.7% (44) | 0.82 (0.54–1.25) | 0.36 | 14.3% (84) | 14.1% (76) | 1.01 (0.74–1.38) | 0.95 |
| Stroke | 2.8% (9) | 1.1% (3) | 2.42 (0.65–8.93) | 0.17 | 2.1% (12) | 1.9% (10) | 1.11 (0.48–2.56) | 0.81 |
| Target vessel revascularization | 11.6% (37) | 16.8% (43) | 0.68 (0.44–1.05) | 0.09 | 10.8% (61) | 13.8% (71) | 0.78 (0.55–1.09) | 0.15 |
| Stent thrombosis (ARC definite or probable) | 4.2% (12) | 7.4% (17) | 0.56 (0.27–1.17) | 0.12 | 5.5% (28) | 5.0% (24) | 1.07 (0.62–1.85) | 0.80 |
| Major bleeding (non–coronary artery bypass related) | 10.9% (37) | 13.3% (36) | 0.81 (0.51–1.28) | 0.37 | 10.7% (64) | 8.7% (48) | 1.25 (0.86–1.82) | 0.24 |
⁎ Any MACEs or major bleeding events, excluding those related to coronary artery bypass grafting.
† Death, reinfarction, ischemic target vessel revascularization, or stroke.
By multivariate analysis, there was no statistically significant difference between abciximab and eptifibatide treatment strategies for NACEs, MACEs, or major bleeding at 30 days and at 3 years ( Table 5 ). A total of 1,342 matched cases (671 each in the abciximab and eptifibatide groups) were found. Similar to the univariate and multivariate results of the total study population, there was no statistically significant difference between the matched groups of abciximab and eptifibatide treatment with regard to MACEs, NACEs, or major bleeding ( Table 6 ).



