17 Common Arterial Trunk or Truncus Arteriosus
I. CASE
A. Fetal echocardiography findings
1. Fetal echocardiography reveals situs solitus of the atria, normal cardiothoracic ratio (0.33), and abnormal cardiac axis of 80 degrees (oriented more toward the left).
2. The heart rate is normal at 143 bpm.
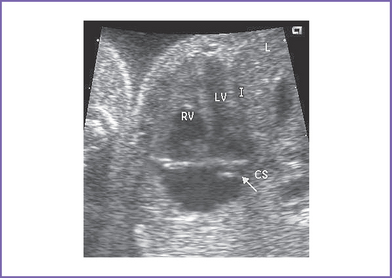
3. The four-chamber view is normal, with balanced ventricles and dilation of the coronary sinus (Fig. 17-1).
4. There is a single arterial trunk with a quadricuspid, dysplastic truncal valve.
a. Mild stenosis (Doppler velocity: 1.3 m/s).
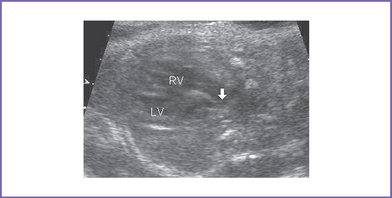
b. Mild truncal valve regurgitation arising from the heart and overriding the crest of the ventricular septum (Fig. 17-2).
5. In the parasternal long axis view of the left ventricle (LV), a moderate to large outlet ventricular septal defect (VSD) is seen.
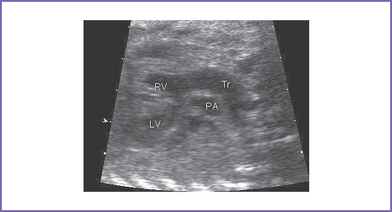
6. Two good-sized branched pulmonary arteries arise from the posterior and leftward aspect of the single arterial trunk (Edwards type II) (Fig. 17-3).
7. The aortic arch is right sided.
8. Myocardial function indices are normal. The right ventricle (RV) and LV Tei indices (myocardial performance index) were normal using the truncal valve.
D. Fetal management and counseling
a. Amniocentesis was done with fetal karyotype and fluorescent in situ hybridization (FISH) for 22q11.2 microdeletion.
b. Results showed normal male karyotype, and FISH was positive for 22q11.2 deletion.
2. Amniocentesis: Diagnosis of tetralogy of Fallot (TOF) should prompt referral for the following:
a. Thorough anatomic examination by ultrasound.
3. Follow-up included serial antenatal studies at 4-week intervals.
a. Degree of truncal valve stenosis and regurgitation and ventricular function were reassessed.
b. Development of hydrops fetalis secondary to congestive heart failure (CHF) is unlikely (approximately 5% risk for all types of congenital heart disease (CHD)), but it could occur if there is progressive truncal valve regurgitation or stenosis.
c. Check for aortic interruption or other associated cardiac anomalies.
E. Delivery
1. In classic truncus without any signs of heart failure, aortic interruption, or other associated malformation, delivery should be as close to term as possible and in an institution with at least neonatal intensive care support.
a. These babies are at high risk for significant transient hypocalcemia.
b. There is also a slight chance the baby will go into heart failure with or without myocardial ischemia (after birth the pulmonary vascular resistance falls and steals from the coronary arteries).
2. Individual management decisions must take into account the level of neonatal support, results of genetic evaluation, parents’ compliance, and the distance from specialized cardiac care.
F. Neonatal management
a. Cyanosis might not be noted immediately at birth.
b. A grade 2/6 systolic murmur with a systolic click is present along the lower sternal border.
c. A high-pitched diastolic murmur of truncal regurgitation might not be present at birth.
d. Signs of CHF can develop within days to weeks of birth as the pulmonary vascular resistance falls.
e. The clinical picture in most infants is that of a large left-to-right shunt with intracardiac mixing, the latter of which results in mild cyanosis.
a. Vigorous anticongestive measures with digitalis and diuretics could be required.
b. Close observation is necessary for evidence of significant diastolic steal, particularly affecting the coronary artery perfusion.
c. Metabolic acidosis, hypoglycemia, hypocalcemia should be treated.
d. Administration of oxygen is usually contraindicated in this disease in the absence of significant pulmonary pathology because it decreases the pulmonary vascular resistance, which would increase pulmonary blood flow further.
e. Given the presence of 22q11.2 microdeletion, the infant will need assessment of T-cell subsets and serum calcium levels. Affected infants often require calcium supplements. Given the potential for immunocompromise, CMV-negative and irradiated blood is critical.
a. Complete correction of common arterial trunk of this type is usually performed in the first 4 to 6 weeks of life. Waiting later than this places the baby at risk for significant pulmonary hypertension.
b. Correction includes closing the VSD, leaving the LV in continuity with the truncal valve, and placing a valved RV–to–pulmonary artery conduit (usually a homograft). The surgeon should attempt to repair the truncal valve, if necessary.
c. The surgical mortality ranges from 2% to 5%.
d. Timing of the operation varies by institution but is generally performed electively at 1 to 3 months of age. Earlier corrective repair may be necessary in a baby with severe heart failure or coronary artery steal.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis is required for life after surgery.
2. For residual hemodynamic abnormalities, anticongestive medications and some restriction of activities may be required.
3. The patient must not engage in competitive sports.
4. For progressive truncal valve regurgitation or stenosis, valve replacement may be needed.
5. RV–to–pulmonary artery conduit replacement will be necessary as the child grows. Cardiac catheterization with intervention may be performed to prolong the interval to reoperation.
6. Atrial and ventricular arrhythmias are possible.
7. Renal dysfunction is possible, given the presence of a single kidney.
8. The 22q11.2 deletion may be associated with:
a. Hypoparathyroidism, necessitating treatment with calcium supplements, at least in early infancy.
b. T-cell subset abnormalities, which can place the infant at risk for certain types of infections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree