High on-treatment platelet reactivity (HTPR) despite clopidogrel therapy is associated with adverse cardiac events after acute myocardial infarction (AMI). Most studies to date have assessed clopidogrel response at a single time point before or after percutaneous coronary intervention (PCI). It is unclear, however, whether the HTPR phenotype is stable over time. Therefore, we aimed to examine response to clopidogrel in patients with AMI treated with PCI over a 6-month period. Patients (n = 57) with AMI treated with PCI were assessed for response to clopidogrel at 3 time points: in hospital, 30 days, and 6 months after index hospitalization. Response to clopidogrel was determined by the VerifyNow P2Y12 assay (reported as P2Y12 response units) and multiple electrode aggregometry (MEA; reported as aggregation units). HTPR was defined as ≥235 P2Y12 response units or ≥47 aggregation units. Patients’ mean age was 54.5 ± 10.9 years, 91% were men, 19% had diabetes, and 74% were admitted with ST-segment elevation MI. HTPR based on MEA was observed in 22.8% of patients in hospital, 26.3% at 30 days, and 17.5% at 6 months (p = NS). HTPR based on the VerifyNow assay was observed in 38.6% of patients in hospital, 28.1% at 30 days, and 33.3% at 6 months (p = NS). Individual HTPR phenotypic assignment at baseline was stable in 73.7% (based on MEA) and 70.2% (based on VerifyNow) of patients at 6-month follow-up. In conclusion, this is the first study evaluating the stability of clopidogrel response over time after AMI. Rates of HTPR to clopidogrel therapy appear to be relatively stable up to 6 months after AMI.
In acute settings of ST-segment elevation myocardial infarction (MI) and non–ST-segment elevation acute coronary syndrome, increased platelet reactivity, increased platelet turnover, and an augmented inflammatory response may be associated with a decreased response to aspirin and clopidogrel. We hypothesized that as time elapses from the acute event, platelet turnover and inflammatory response would subside and, hence, response to aspirin and clopidogrel would improve. Accordingly, our aim was to evaluate and follow up to 6 months the response to aspirin and clopidogrel in patients with acute MI (AMI).
Methods
Patients who presented with ST-segment elevation MI or non–ST-segment elevation acute coronary syndrome and were treated with percutaneous coronary intervention (PCI) at the Rabin Medical Center, Israel were eligible for participation in the study. All patients received aspirin 100 mg at time of presentation, followed by 100 mg/day. A loading dose of clopidogrel 600 mg was administered preferably before the procedure (if not, immediately after PCI), followed by 75 mg/day. Adjunctive treatment during PCI (heparin, bivalirudin, and/or glycoprotein IIb/IIIa inhibitors) was given according to the physician’s discretion and preference and was documented in all patients. Specific exclusion criteria to enrollment included any contraindications for using aspirin or clopidogrel, concurrent treatment with warfarin, severe bleeding diathesis, thrombocytopenia (<100 × 10 3 cells/mm 3 ), anemia (hemoglobin <10 g/dl), renal insufficiency (creatinine >2.5 mg/dl), and history of a major co-morbid illness that would prevent participation during the full duration of the study. Furthermore, any patient scheduled to discontinue clopidogrel before final follow-up at 6 months was excluded from enrollment. The study was approved by the investigational review board (Helsinki committee) of the Rabin Medical Center, and all subjects provided written informed consent.
All patients were treated from the day after PCI for ≥6 months with aspirin 100 mg/day and clopidogrel 75 mg/day. All blood samples for platelet function testing were drawn from an antecubital vein using a 19-gauge needle. Samples were taken after fasting from midnight and in a state of rest. For each sample, blood was collected in tubes containing 3.2% citrate. Tubes were filled to capacity and then gently mixed. Blood samples were processed within 1 hour of blood collection. Four blood samples were drawn over the 6-month study period: (1) 18 to 24 hours after index PCI, (b) at 3 to 4 days of hospitalization and before discharge, (c) 30 days after hospitalization (and MI), and (4) at 6 months after hospitalization (and the MI).
Platelet responsiveness to antiplatelet therapy was assessed using 2 systems: multiple electrode aggregometry (MEA) and the VerifyNow P2Y12 assay. The multiple electrode aggregometer (Dynabyte, Munich, Germany) is an impedance aggregometer that tests platelet function in whole blood. After diluting the sample with normal saline (0.9%), adenosine diphosphate (ADP) 6.4 μmol/L (to evaluate clopidogrel response) and arachidonic acid 0.5 mmol/L (to evaluate aspirin response) were added to separate test cuvettes. Platelet aggregation was recorded as aggregation units continuously for ≥6 minutes. Area under the curve was calculated and expressed as aggregation units multiplied by minutes. VerifyNow P2Y12 (Accumetrics, San Diego, California) is a rapid platelet-function cartridge-based assay designed to directly measure drug effect on the P2Y12 receptor. The assay contains ADP 20 μmol and prostaglandin E1 22 nmol to decrease the activation contribution from ADP binding to P2Y1 receptors. Fibrinogen-coated microparticles are used in the VerifyNow P2Y12 cartridge to bind to available platelet receptors. The VerifyNow instrument measures platelet-induced aggregation as an increase in light transmittance and is expressed in P2Y12 reaction units. With this assay, a higher P2Y12 reaction unit reflects greater ADP-mediated platelet reactivity.
The primary end point of the study was the rate of the high on-treatment platelet reactivity (HTPR) phenotype for clopidogrel response, defined by ADP-induced MEA and the VerifyNow P2Y12 assay, at each time point during follow-up. Secondary end points include mean platelet function parameters at each time point. Standard definitions established by a recent consensus document were used to define clopidogrel HTPR. Based on MEA, a cut-off value of 47 aggregation units was used to define HTPR for clopidogrel treatment. Based on the VerifyNow P2Y12 assay, HTPR was defined as ≥235 P2Y12 reaction units.
Because most patients analyzed in this study received perioperative eptifibatide (75.4%), inhibition of glycoprotein IIb/IIIa may have confounded the antiplatelet effects of aspirin and clopidogrel at the early point (24 hours after PCI). Therefore, in the present analysis, only the predischarge time point (3 to 4 days after PCI) was taken into account and included in the comparisons of HTPR rates.
All platelet function parameters assessed during the study period were found to be normally distributed by the Kolmogorov–Smirnov test. Continuous variables are presented as mean ± SD. Comparison of mean platelet function measurements across various time points was performed by 2-factor analysis of variance with repeated measurements. When the interaction between the repeated-measurements factors was significant, post hoc analysis for specific pairwise comparisons was performed by Newman–Keuls tests. Comparisons between categorical variables were performed using chi-square tests. The interaction terms between important prespecified clinical variables (ST-segment elevation MI/non–ST-segment elevation acute coronary syndrome and diabetes) and platelet function over time were found to be not significant and thus further subgroup analyses were not undertaken. Analyses were performed using SPSS 11.0 (SPSS, Inc., Chicago, Illinois) and statistical significance was set at a p value ≤0.05.
Results
Fifty-seven patients were recruited who underwent urgent or emergency PCI for AMI. Of the final study participants, 42 (73.7%) developed ST-segment elevation MI and 15 (26.3%) were admitted with non–ST-segment elevation MI. Demographic characteristics, co-morbid conditions, and procedural details for all patients are presented in Table 1 . Study participants had a mean age of 54.5 ± 10.9 years and were predominantly men (91.2%) and overweight (mean body mass index 27.5 ± 3.2 kg/m 2 ). Major co-morbid conditions included hyperlipidemia (57.9%) and hypertension (45.6%). Only 9 patients (15.8%) had previously undergone PCI.
| Age (years) | 54.5 ± 10.9 |
| Men | 52 (91%) |
| Body mass index (kg/m 2 ) | 27.5 ± 3.2 |
| Diabetes mellitus | 11 (19%) |
| Hypertension ⁎ | 26 (45%) |
| Hyperlipidemia † | 33 (58%) |
| Current or former smoker | 22 (39%) |
| Family history of coronary artery disease | 13 (23%) |
| Previous myocardial infarction | 10 (18%) |
| Previous coronary artery bypass graft surgery | 4 (7%) |
| Previous percutaneous coronary intervention | 9 (16%) |
| ST-segment elevation myocardial infarction | 42 (74%) |
| Non–ST-segment elevation acute coronary syndrome | 15 (26%) |
| Periprocedural eptifibatide | 43 (75%) |
⁎ Blood pressure ≥140/90 mm Hg at enrollment or use of antihypertensive medication.
† Total cholesterol ≥200 mg/dl at enrollment or use of lipid-lowering medication.
Before presentation, 22 patients (38.6%) were taking aspirin and only 2 (3.5%) were taking clopidogrel. All 42 patients with ST-segment elevation MI received the clopidogrel loading dose before PCI. Of the 15 patients with non–ST-segment elevation MI, 11 received the clopidogrel loading dose before PCI and 4 immediately after. At discharge, all patients received a statin and an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (in addition to aspirin and clopidogrel). Most patients were also discharged on a β blocker.
Rates of in-hospital HTPR despite clopidogrel therapy were 22.8% (defined by MEA–ADP test) and 38.6% (defined by VerifyNow P2Y12 assay). These rates were not significantly different at 30 days and 6 months after index hospitalization for AMI. Figure 1 shows the time course of overall rates of HTPR defined by MEA and the VerifyNow P2Y12 assay.
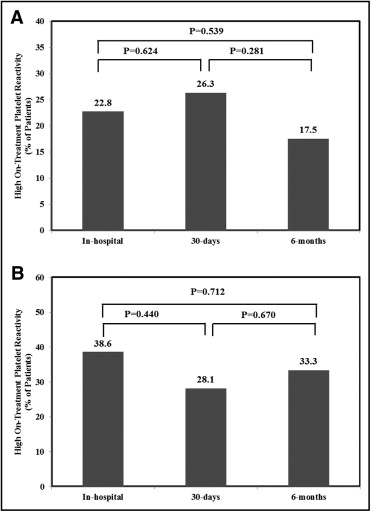
Table 2 lists temporal trends of mean platelet function values over time after AMI in response to dual antiplatelet therapy. MEA stimulated by arachidonic acid at baseline was 17.6 ± 1.7 aggregation units and did not significantly change during the follow-up period. MEA in response to ADP at baseline (in hospital) was 31.6 ± 2.2 aggregation units and remained stable at 30 days (32.8 ± 2.7 aggregation units). At 6-month follow-up, aggregation significantly decreased to 27.5 ± 2.3 aggregation units (pairwise comparison 30 days vs 6 months, p = 0.0004). Platelet reactivity, determined by the VerifyNow P2Y12 assay, at baseline (in hospital) was 207.2 ± 10.6 P2Y12 reaction units. These values did not significantly change across study time points ( Table 2 ).



