11 Clinical Trials of Defibrillator Therapy
 Sudden Death: Definition and Implication for Clinical Trials
Sudden Death: Definition and Implication for Clinical Trials
Sudden cardiac death is defined as a death attributable to cardiac cause that occurs soon after the onset of symptoms. A 49% reduction in the incidence of sudden death has been documented over more than 50 years.1 The incidence of out-of-hospital cardiac arrest from ventricular fibrillation (VF) has also been reduced.2 These reductions largely appear to be related to improved management of coronary heart disease risk factors and prevention strategies that include prophylactic insertion of ICD systems.3
Despite these advances, sudden death remains a major public health problem. Worldwide, more than 3 million lives are ended prematurely because of sudden death,4 many in ambulatory populations from sustained ventricular tachycardia (VT) or VF.5,6 Bradyarrhythmic events and electromechanical dissociation may be more common mechanisms in patients with end-stage heart failure.7
A definition of sudden death that includes events that occur within 1 hour of symptom onset has been considered reliable in identifying deaths related to arrhythmias.8 Definitions of sudden death that also incorporate the circumstances surrounding these events may provide additional value in separating arrhythmic from nonarrhythmic causes.9 Despite these refinements, however, misclassification occurs.10 Autopsy data from a large study of patients who survived a myocardial infarction (MI), had residual left ventricular (LV) dysfunction, and later suffered sudden death found that half of deaths categorized as “sudden” were nonarrhythmic. Most of these nonarrhythmic sudden deaths were related to a recurrent MI or cardiac rupture and were more frequent in the initial 6 months after the initial MI.11 Another study found that VT or VF were responsible for most sudden deaths in a group of post-MI patients with moderate LV dysfunction in whom the cardiac rhythm was monitored at the time of these events. Bradyarrhythmias were responsible for a minority of these sudden arrhythmic deaths, and VT or VF was the underlying rhythm for some deaths categorized as nonarrhythmic.6
 Evolution of Therapy: Implication for Clinical Trials
Evolution of Therapy: Implication for Clinical Trials
The first implantation of an automated defibrillator occurred in the 1970s.12 Over the past three decades, improvements have been made in device capability and size. The contemporary tiered-therapy ICD is capable of delivering antitachycardia pacing (ATP), low-energy cardioversion, and high-energy defibrillation therapies. In addition, it provides backup bradycardia pacing. The ICD effectively terminates the vast majority of sustained VT or VF events and most bradyarrhythmias.13 Enhancements that include painless ATP therapies,14,15 algorithms to reduce the likelihood of unnecessary ICD therapies,16 and remote monitoring17,18 have added to the appeal of the ICD. The addition of cardiac resynchronization therapy (CRT) offers the potential to improve LV function, reduce morbidity, and further decrease mortality in select patients.19
Most contemporary ICD systems are placed in a subcutaneous or submuscular position in the chest and use the venous anatomy for lead delivery. The risk of death related to placement of nonthoracotomy, transvenous ICD systems approaches 0%. In contrast, earlier ICD systems that required a thoracotomy or sternotomy for lead placement were associated with a 5% mortality risk in the first 30 postoperative days.20,21 The ease of implantation and relatively low risk of surgical morbidity and mortality with contemporary ICD system placement have altered the direction of studies examining ICD efficacy. Initial trials were limited to patients considered at “very high risk” of cardiac arrest, whereas later trials have included patients at somewhat lower risk.
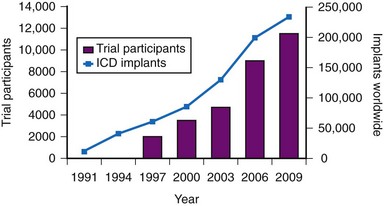
The results of clinical trials over the past two decades have established the ICD as a cornerstone of therapy for patients at risk for sudden death.22,23 The number of ICD implantations worldwide now exceeds 250,000 per year.24 The exponential increase in the number of ICD systems implanted over the past decade mirrors the quantity of patient data obtained from randomized clinical trials4,24 (Fig. 11-1).
 Quality of Life
Quality of Life
In contrast to antiarrhythmic drugs, which are designed to prevent arrhythmias, the ICD treats arrhythmias that have already developed. Because the ICD prolongs life, patients may experience deterioration in underlying health that might not have otherwise occurred. Stress, anxiety, depression, mood disturbance, sexual dysfunction, and a sense of uncertainty or loss of control have been described in ICD recipients.25 The ICD also provides a sense of reassurance to some patients, enhancing their quality of life (QOL). The use of an ICD with CRT may further enhance QOL by reducing heart failure symptoms and preventing hospitalizations.19
Six large trials have published data related to QOL with ICD therapy. Two trials included patients with spontaneous or inducible ventricular arrhythmias and found that ICD therapy and amiodarone were associated with similar alterations in QOL.26,27 Results from the four trials that included patients without a history of these arrhythmias are less consistent. The Coronary Artery Bypass Graft (CABG) Patch trial found reduced QOL with ICD therapy,28 as did the Second Multicenter Automatic Defibrillator Implantation Trial (MADIT-II).29 In contrast, the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial30 and the Sudden Cardiac Death Heart Failure Trial (SCD-HeFT) found no significant difference in QOL over time.31 Details regarding the QOL assessment methods and potential explanations for these differences are provided later in the individual reviews of these trials.
 Limitations of Therapy
Limitations of Therapy
Shocks
Even sporadic ICD shocks are associated with significant, independent reductions in QOL. Compared with patients who did not experience shocks, patients in the Antiarrhythmics versus Implantable Defibrillators (AVID) trial who experienced at least one shock during the initial year of follow-up had reduced QOL, independent of LV ejection fraction (EF), social circumstances, and medication use.26 The reductions in QOL associated with shocks in AVID were similar in magnitude to clinically important adverse effects from amiodarone. In MADIT-II, a higher prevalence of heart failure and the development of shocks in ICD-treated patients were associated with reductions in QOL among ICD recipients.29 ICD shocks were also associated with reduced QOL in the DEFINITE trial.30 In SCD-HeFT, QOL was diminished over the short term following ICD shocks, but subsequently improved.31 This implies a direct association between ICD shocks and reduced QOL.
The development of clusters of life-threatening arrhythmias identifies patients at high risk of death in the near term. Patients in AVID who experienced electrical storm (at least three episodes of VT or VF in a 24-hour period) had a greater than fivefold higher risk of death in the subsequent 3 months.32 The majority of these deaths are related to progressive heart failure, supporting a link between electrical storm and deteriorating LV function. The MADIT-II investigators found that inappropriate ICD shocks were common, representing over 30% of all shocks, and were associated with a twofold higher risk of death. Most of these inappropriate shocks were related to atrial fibrillation or a supraventricular tachycardia.33 In SCD-HeFT, risk of death with appropriate ICD shocks was fivefold higher, and risk of death with inappropriate ICD shocks was twofold higher. Most of the deaths in the patients who received shocks and later died in SCD-HeFT were attributed to progressive heart failure.34
It is not known whether the development of isolated or multiple ICD shocks identifies patients with worsening LV function or whether the ICD therapies themselves contribute to progressive LV dysfunction. Evidence links multiple shocks with myocardial injury and fibrosis.35,36 There is also evidence in animal models that ICD shocks may be proarrhythmic.37 Data from human studies support a proarrhythmic effect of some shocks and suggest that multiple shocks can result in myocardial stunning and electromechanical dissociation.38,39 Also, observational data indicate that ICD shocks, but not ATP therapies, are associated with a higher risk of death.40 However, other studies did not find a difference in outcome if arrhythmias were terminated by ATP or by shocks.32 Nonetheless, strategies to reduce the likelihood of inappropriate and appropriate shocks, and alternative methods to terminate ventricular arrhythmias, are vital avenues of investigation.16
Antiarrhythmic drugs can be used to reduce the likelihood of ICD shocks,41,42 but may negatively affect QOL because of their adverse effects.26 ATP therapies painlessly interrupt reentrant ventricular arrhythmias using brief, rapid bursts of overdrive pacing. ATP has long been used to terminate slower ventricular arrhythmias (rates <188 beats per minute [bpm]) with an efficacy exceeding 90% and a low (<5%) risk of accelerating these slower arrhythmias to a more rapid one that requires a defibrillation shock. ATP has also been shown to be useful for terminating fast VT (188-250 bpm)43 and should be used as the initial treatment for treating these arrhythmias.16 The use of ATP for fast VT has been demonstrated to enhance QOL,43 both in patients with, and in those without, a history of sustained arrhythmias before ICD implantation.44 Extending the time to detect and treat a ventricular arrhythmia, from 4 to 5 seconds to 9 to 12 seconds, also appears to be useful in reducing the number of ICD shocks,45 because even fast VT episodes will spontaneously terminate 30% of the time.43 The combined use of algorithms that avoid shocks from external and intracardiac noise, provide enhanced redetection, and maximize the use of ATP therapies has been estimated to reduce unnecessary ICD shocks by more than 80%.46 It is important to note that the safety and efficacy of delaying the detection of these arrhythmias or the use of multiple algorithms to reduce shocks are not known. Ongoing studies are in progress to address these and other relevant issues.
Reliability
Despite the intuitive appeal of the ICD, the clinician must recognize that it is an imperfect therapeutic device. Present ICD systems have a limited working life and are subject to both complications and unexpected failure over time.47–49 However, the life expectancy of patients receiving ICD systems has improved over time. One study found that 75% of ICD recipients were alive at 5 years and more than 40% were alive at 10 years after ICD placement.48 This is consistent with a 49% survival rate after a median follow-up of over 7 years in MADIT-II ICD recipients.50 A contemporary ICD battery is anticipated to last 4 to 7 years, but clinical experience is that the average in-service time for an ICD is typically 3 to 5 years due to premature battery depletion, device advisories, need for system upgrades, and infections.51 Therefore, recipients are likely to require multiple devices over their lifetime. With recent trials showing benefit in patients with less advanced forms of heart disease, this mismatch will continue to grow. Research related to assessing reliability and longevity is required. Longer-term follow-up of ICD patients in clinical trials is also needed to obtain reliable data on long-term efficacy and cost-effectiveness of ICD therapy.
Cost
Cost-effectiveness is crucial in the setting of limited or restricted health care resources. This is particularly relevant as use of ICD and CRT devices expands to include patients sharing only some similarities with patient populations in whom these devices have been shown to be effective, so-called indication creep or indication extrapolation.52 For example, a large survey of 141 centers in Western Europe found that at least 1 in 5 patients undergoing a CRT procedure had clinical or electrocardiographic characteristics that placed them outside of published guidelines.53 Analysis of data from the National Cardiovascular Data Registry (NCDR) ICD Registry similarly found that over 20% of patients receiving CRT devices did not meet guideline-based indications.54
A major limitation of cost analyses in randomized trials is that these analyses invariably inflate the cost of ICD therapy and devalue the life-years gained. The estimate of life-years gained from randomized trials depends heavily on the duration of follow-up assessing benefit. The cost of an ICD is heavily front-loaded, yet its benefit accrues over time. Thus, short-term data from randomized trials may artificially underestimate the cost-effectiveness of ICD therapy.55 For example, the mortality reduction observed with long-term follow-up in MADIT-II was much larger than that initially observed, despite many patients in the conventional group crossing over to ICD therapy at the conclusion of the trial.50 At a median follow-up of over 7 years, the absolute reduction in mortality with ICD therapy was 13%. This is much larger than the 5.6% reduction in mortality at the trial’s conclusion, where median follow-up was 1.5 years.56
An in-depth analysis of randomized trials of ICD therapy found a modest, incremental cost-effectiveness of ICD therapy that ranged between $25,000 and $50,000 per quality-adjusted life-year.57 Similar estimates have been found in most randomized trials. These data indicate that ICD therapy is cost-effective. However, it is important to recognize that cost-effectiveness likely differs in the real world versus the populations from whom these estimates were derived. For example, only sparse data exist on the cost-effectiveness of ICD therapy in elderlypersons.58 Additional data regarding cost-effectiveness are included in the discussion of individual trials.
Utilization
There are marked differences in the use of ICD therapy in different regions of the world. In the United States, the rate of ICD implantations is approximately 600 implants per 1 million population. In Western Europe and Canada, the rates are approximately one quarter of the U.S. rate.24 The reasons for these differences are complex, but likely relate to differing perceptions of need, variability in guidelines, perceived cost-effectiveness, and concerns over inappropriate ICD therapies and device malfunction.59 This underutilization has also been attributed to a shortage of electrophysiologists, poorly developed local referral strategies and care pathways, and difficult or confusing financial circumstances.24 Solutions to some of these barriers are more obvious than others. For example, an entirely subcutaneous ICD system may address limited implantation resources.60 However, issues then arise related to how these patients and devices will be followed. Studies assessing the long-term effectiveness and safety of subcutaneous ICD systems are in progress. Underuse of ICD therapy is concerning and has significant consequences for patients in whom an ICD is clearly indicated, but not prescribed. Additional efforts to identify and address barriers to patient referral and implantation rates are required.
 Groups at Risk for Sudden Death
Groups at Risk for Sudden Death
Identifying Individuals at Risk
A major barrier to preventing sudden death is the lack of accurate and reliable methods of identifying the majority of individuals at risk and those in whom an ICD would be most beneficial.61 Individuals who can be identified before experiencing sudden death can be categorized as belonging to one of the four groups previously listed. Patients with spontaneous or inducible ventricular arrhythmias are known to be at high risk for cardiac arrest, but they represent only a fraction of those who will experience sudden death62,63 (Fig. 11-2).
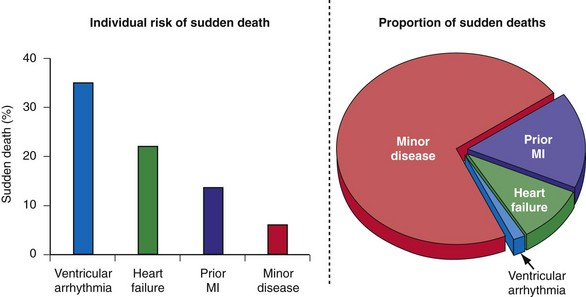
Figure 11-2 Sudden death: individual risk versus population impact.
(Modified from Rea TD, Pearce RM, Raghunathan TE, et al: Incidence of out-of-hospital cardiac arrest. Am J Cardiol 93:1455-1460, 2004.)
Patients with structural heart disease, notably a previous MI or LV dysfunction, represent a significant proportion of those at risk for sudden death and have an intermediate risk of cardiac arrest in the near term. To date, only two approaches have proved useful in guiding prophylactic ICD therapy in this group. LV dysfunction alone (LVEF ≤ 0.35) or in combination with the induction of sustained VT/VF with invasive programmed electrical stimulation.56,64,65 However, invasive programmed electrical stimulation does not appear useful early after MI,66,67 and arrhythmia inducibility is unreliable in predicting long-term outcome.68
Although individuals with LV dysfunction after MI have a heightened risk of sudden death, most will not experience a cardiac arrest in the near term. Further, the majority of sudden deaths occur in post-MI patients with better-preserved LV function69,70 (Fig. 11-3). If a left ventricular ejection fraction cutoff value of 0.35 or less (LVEF ≤ 0.35) is used to identify those at risk, fewer than one third of patients who will suffer sudden death are identified.69–72 In contrast, a cutoff of 0.50 or less (LVEF ≤ 0.50) will identify most patients at risk.69,70 However, an LVEF ≤ 0.50 alone is a nonspecific selection method, and additional risk assessment is required to better identify those at risk for sudden death.
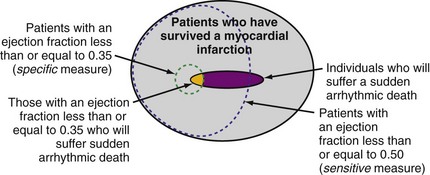
Figure 11-3 Identifying individuals at risk for sudden death: specificity versus sensitivity.
(Modified from La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 351:478-484, 1998; and Exner DV, Kavanagh KM, Slawnych MP, et al: Noninvasive risk assessment early after a myocardial infarction: the REFINE study. J Am Coll Cardiol 50:2275-2284, 2007.)
Individuals with mild or subclinical heart disease account for the majority of sudden deaths,63,73 but the likelihood that an individual in this group will experience a cardiac arrest over a decade is very low (see Fig. 11-2).74 Many of the cardiac arrests in this group appear to be triggered by unstable coronary artery plaques.75 Appropriate attention to therapy with antiplatelet agents, statins, fish oils, and other drugs has the potential to significantly reduce the burden of sudden death.76 The widespread use of automated external defibrillators (AEDs) has been advocated as one method to improve survival from sudden death, but the Home AED Trial (HAT) found that an AED plus cardiopulmonary resuscitation (CPR) offered no advantage over CPR alone in patients at risk for sudden death because of a history of prior MI.77 The efficacy of a wearable AED in patients considered at risk for sudden death, but who are not candidates for an ICD (e.g., LV dysfunction early after MI), is presently under investigation.
Noninvasive methods to assess the location and extent of myocardial scarring,78 cardiac denervation,79 ventricular conduction, cardiac repolarization, and autonomic tone61,80 have been developed to identify patients at risk for sudden death. However, although noninvasive techniques such as T-wave alternans (TWA), heart rate turbulence (HRT), other Holter monitor indices, imaging methods, and genetic assessment may be useful in some patients, these methods have not been useful in identifying groups of patients likely to benefit from an ICD.
The signal-averaged electrocardiogram (signal-averaged ECG) was considered a reliable means to identify patients at risk for sudden death, but failed to predict ICD efficacy in the CABG Patch study.81 Similarly, heart rate variability was regarded as a reliable marker for predicating sudden death after MI, but no survival benefit was identified with ICD therapy in the Defibrillators in Acute Myocardial Infarction Trial (DINAMIT).82 The Microvolt T Wave Alternans Testing for Risk Stratification of Post–Myocardial Infarction Patients (MASTER) study, although not randomized, found that microvolt TWA results were not helpful in identifying post-MI patients with LV dysfunction at risk for arrhythmic death or in predicting ICD effectiveness.83
Relative VS. Absolute Risk Reduction and Number Needed to Treat
When assessing the impact of a therapy, one must consider its relative and absolute benefit. The absolute risk reduction is the difference between control and intervention group rates. For example, ICD therapy reduced mortality by 5.6% (19.8% control vs. 14.2% ICD) after a median follow-up of 1.5 years in MADIT-II. At a median follow-up of more than 7 years, the absolute risk reduction was 13% (62% control vs. 49% ICD).50 The relative risk reduction is the percentage reduction in rates with the intervention. For this same example, the relative risk reduction at 1.5 years in MADIT-II was 31% (5.6% absolute reduction ÷ 19.8% control rate) and at 7 years was 21% (13% absolute reduction ÷ 62% control rate). The number of patients needing to be treated in order to prevent one event is often used to describe the absolute impact of a therapy. The number needed to treat (NNT) is the reciprocal of the absolute risk reduction. In MADIT-II the NNT after 1.5 years was 17.9 (100% ÷ 5.6%) and at 7 years was 7.7 (100% ÷ 13%). This information is important for clinical decision making and health policy.
 Evolution of ICD Therapy
Evolution of ICD Therapy
Initial observational studies and smaller randomized trials suggested that ICD implantation was associated with a greater than 60% relative reduction in mortality compared with conventional strategies.84 Because of limitations in the design of these initial studies, a series of larger, adequately powered, randomized trials were conducted either to confirm or to refute these initial observations. Because of the risk of ICD system placement (via thoracotomy or sternotomy), initial randomized trials focused on patients with the highest individual risk for a fatal cardiac arrest, those with a history of spontaneous ventricular arrhythmias (see Fig. 11-2). The next group of studies evaluated patients with an extremely high risk of a fatal cardiac arrest caused by a history of a prior MI, severe LV dysfunction, and arrhythmias inducible by invasive programmed electrical stimulation. During this time, studies were also designed to minimize the risk of ICD system implantation by limiting therapy to patients who required a sternotomy for another reason (e.g., CABG surgery).81 After the mostly positive results of these early trials, studies in populations with lower but significant risk of sudden death were initiated. These trials have mostly been limited to patients with heart failure and LV dysfunction.
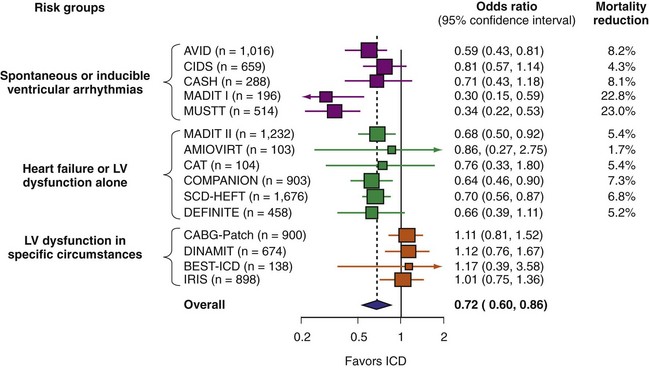
A number of randomized studies have compared ICD therapy with amiodarone or other medical therapy. The relative benefit of ICD was consistent in most of these studies. However, no benefit of ICD therapy was found in four adequately powered trials (Fig. 11-4). These four trials included patients with LV dysfunction in specific circumstances. Three studies enrolled patients very early after MI,82,85,86 and the other included patients undergoing CABG surgery.81 The lack of ICD efficacy in these populations can be explained by (1) post-MI coronary artery revascularization and remodeling dramatically reducing the risk of sudden death, thus reducing ICD efficacy,87 or (2) use of inadequate risk assessment methods to select patients. Given the utility of most of these tests,80 the second explanation appears less likely. The neutral results from these four trials emphasize the need for randomized trials to assess ICD efficacy definitively in new populations and highlight the pitfalls of using a therapy for an indication beyond what has been shown to be efficacious.
 Efficacy: Review of Randomized Trials
Efficacy: Review of Randomized Trials
Patients with Spontaneous or Inducible Ventricular Arrhythmias
Table 11-1 summarizes the inclusion criteria, primary endpoints, and main results for five studies involving patients with spontaneous or inducible ventricular arrhythmias. Table 11-2 presents details of the study populations, risk of the untreated population (annual control group mortality), and relative and absolute changes in mortality with ICD therapy.
TABLE 11-1 Clinical Trials of ICD Therapy for Spontaneous or Inducible Ventricular Arrhythmias: Inclusion Criteria, Comparison Groups, and Main Results
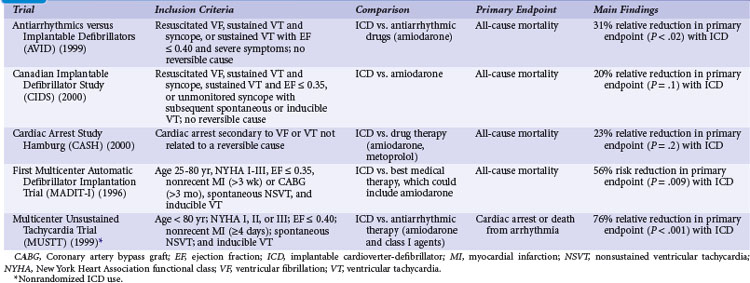
TABLE 11-2 Clinical Trials of ICD Therapy for Spontaneous or Inducible Ventricular Arrhythmias: Population Details and Mortality Results
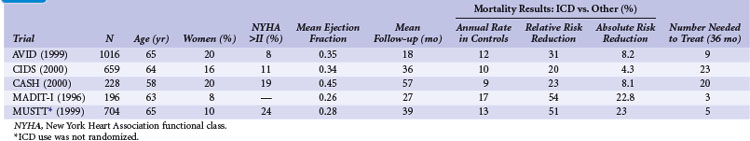
Two large randomized trials88,89 and one small trial90 provide convincing evidence that ICD therapy is superior to class III antiarrhythmic drugs in reducing mortality among patients with mostly spontaneous life-threatening arrhythmias. These studies generally included patients who survived a cardiac arrest caused by VT or VF. Another small randomized trial compared ICD therapy with conventional care that generally included amiodarone,64 and a large randomized trial in which ICD therapy was used in a nonrandomized manner91 assessed the efficacy of ICD therapy in patients with LV dysfunction and inducible ventricular arrhythmias. Both trials showed a benefit from ICD therapy similar to that in the three studies of patients who survived a cardiac arrest.
Antiarrhythmics versus Implantable Defibrillators Trial
The Antiarrhythmics versus Implantable Defibrillators (AVID) trial was a large (n = 1016) multicenter trial conducted in the United States and Canada. Patients entered into the trial were resuscitated from VT, had sustained VT with syncope, or had sustained VT with an LVEF of 0.40 or less and symptoms suggesting severe hemodynamic compromise (near-syncope, heart failure, and angina).88 Patients were excluded if the ventricular arrhythmia was attributed to a reversible cause or if the episode of VT was categorized as stable. The LVEF was required to be ≤0.40 in patients who underwent revascularization after the index arrhythmia, regardless of the qualifying arrhythmia. The primary outcome of AVID was all-cause mortality.
The AVID trial began recruitment in mid-1993 and was terminated in 1997, when patients randomly assigned to ICD therapy were found to have a 31% relative reduction in the risk of death compared with patients receiving antiarrhythmic drug therapy, mostly amiodarone (95% confidence interval [CI] = 10% to 52%). This mortality benefit translates into an average life extension of 2.7 months during 18 months of follow-up.92 The NNT over 36 months is approximately 9 (11.3% absolute risk reduction) and the incremental cost of an ICD at 3 years was approximately $67,000 (U.S.) per life-year saved. However, the early termination of the AVID trial has two important implications: (1) premature termination may overestimate the magnitude of benefit with ICD therapy compared with amiodarone,93 and (2) the resultant shorter average duration of follow-up may lead to underestimation of the true cost-effectiveness of ICD therapy.94
Analyses were undertaken to identify groups more likely to benefit from ICD therapy. The 61% of patients with LVEF values less than 0.35 had a large (40%) relative reduction in the risk of death with ICD compared with drug therapy, whereas patients with higher values did not significantly benefit.95 AVID included relatively few patients with advanced heart failure, marked reductions in LVEF, or advanced age and thus had limited power to assess the relative efficacy of ICD therapy versus amiodarone in these and other subgroups.96
As a prespecified secondary endpoint, the AVID investigators also collected QOL data before and at 3, 6, and 12 months after randomization.26 Data were available for 905 of 1016 (89%) patients enrolled. Of these, 800 patients survived 1 year or longer and constituted the QOL study population. Moderate to severe impairment in both physical functioning and mental well-being were evident in both treatment groups at baseline. Patients who received an ICD had better physical functioning over the subsequent year, but no significant changes were noted in mental well-being. No significant difference in QOL over time was observed between the ICD-treated and amiodarone-treated patients. Adverse symptoms were associated with a decrease in QOL in both groups. As previously discussed, there was an independent association between ICD shocks and reduced QOL. The reduction in QOL with multiple ICD shocks was of similar magnitude to the reduction in QOL observed among patients with severe adverse symptoms from amiodarone.
One criticism of AVID was an imbalance in β-blocker use between patients assigned to an ICD (38% at 1 year) versus amiodarone (11% at 1 year). However, β-blocker use did not alter survival among those treated with an ICD or amiodarone. In contrast, patients who were eligible for the AVID trial, but were not randomly assigned to and did not receive amiodarone or an ICD, had a 53% lower risk of death with a β-blocker versus similar patients who did not receive β-blockers.97 These data suggest that β-blockers may reduce sudden death, but this effect is mitigated with the use of an ICD or amiodarone.
Another intriguing result from AVID was that patients with sustained VT or VF caused by a reversible cause had a similar, or perhaps higher, risk of death compared with patients in whom the event was considered a primary episode of VT or VF.98 A separate analysis found that the prognosis of patients with stable VT was similar to those with unstable VT.99 These analyses question the dogma that patients with stable VT and those with a potentially reversible cause of sustained VT or VF have a good prognosis and are not candidates for an ICD.
Canadian Implantable Defibrillator Study
The Canadian Implantable Defibrillator Study (CIDS) was a large (n = 659) multicenter trial conducted in Canada, Australia, and the United States from 1990 to 1997.89 Patients were eligible if they had VF, sustained VT causing syncope, sustained VT and an LVEF ≤ 0.35, or unmonitored syncope with subsequent spontaneous or inducible VT. Patients who had experienced a recent MI (within 72 hours) or had an electrolyte imbalance were excluded. The average follow-up in CIDS (36 months) was twice that of AVID, but the number of deaths was somewhat fewer in CIDS (181) than in AVID (202). The primary outcome in CIDS was all-cause mortality.
This study demonstrated a 20% lower relative (95% CI = 8% increase to 40%) risk of death with an ICD than with amiodarone (P = .14). On the basis of this 4.3% absolute reduction in mortality, the NNT over 36 months was 23. A subsequent single-center analysis of 120 patients found a high rate of cessation of amiodarone because of adverse effects (82% of patients) and a reduction in mortality with an ICD (16 deaths; 27% mortality) versus amiodarone (28 deaths; 47% mortality) over 11 years of follow-up.100
The incremental cost of an ICD was approximately $150,000 (U.S.) per life-year gained.101 Patients in CIDS with low LVEF values benefited from ICD therapy to a greater extent than those with better-preserved LV function. The selective use of an ICD for patients with LVEF values less than 0.35 lowers the incremental cost of an ICD to less than $70,000 per life-year gained.102 Another analysis demonstrated that patients with two or more of three characteristics that included age 70 years or older, LVEF ≤0.35, and New York Heart Association functional class (NYHA Class) greater than II benefited from ICD therapy, whereas patients with one or no characteristics did not.103 However, the CIDS risk score was imperfect when applied to AVID.104
Quality of life was assessed in CIDS using the Rand Corporation’s 38-item Mental Health Inventory and the Nottingham Health Profile.27 Of the initial 400 enrolled patients, only 178 (45%) provided analyzable QOL data at 1 year. Patients randomly assigned to ICD therapy had significant improvements in several QOL measures over 1 year of follow-up. In patients undergoing amiodarone therapy, these QOL measures either did not improve or deteriorated. Patients with at least five ICD shocks during follow-up had reduced QOL. Whether ICD therapy truly provides better QOL than amiodarone therapy is uncertain because of the conflicting results from the AVID trial and the methodologic issues related to the CIDS analysis.105
Cardiac Arrest Study Hamburg
The Cardiac Arrest Study Hamburg (CASH) was a smaller randomized trial (n = 288) that compared the use of an ICD with drug therapy.90 Patients were enrolled from 1987 to 1996. A significant number of patients underwent a thoracotomy or sternotomy for ICD system placement (before July 1991). The study population included patients resuscitated from a cardiac arrest secondary to a documented sustained VT or VF. Patients were excluded if the cardiac arrest occurred within 72 hours of an MI or cardiac surgery or was related to an electrolyte abnormality or proarrhythmic drug. The primary endpoint was all-cause mortality.
Pooled Analysis of the Three Studies
The combined results of the AVID, CIDS, and CASH data demonstrate that mortality is reduced by 27% (95% CI = 13% to 40%; P <.001) with an ICD compared with amiodarone.22 Death attributed to an arrhythmia was reduced by 51% (95% CI = 33% to 64%; P < .001). No significant difference between treatment groups in the risk of death from nonarrhythmic causes was evident. This reduction in mortality corresponded to an average extension in life of 2.1 months. The annual mortality with an ICD was 12.3% per year, versus 8.8% per year with amiodarone. The absolute reduction in mortality (10.5%) over 36 months translates into needing to treat fewer than 10 patients in order to prevent one death.
First Multicenter Automatic Defibrillator Implantation Trial
The First Multicenter Automatic Defibrillator Implantation Trial (MADIT-I) was a small (n = 196) multicenter trial conducted in the United States, Germany, and Italy from December 1990 to March 1996.64 The first one half of the patients received epicardial ICD systems, and the remaining half received nonthoracotomy, transvenous systems. ICD therapy was compared with conventional care, mostly empiric amiodarone therapy, in patients with ischemic LV dysfunction, asymptomatic nonsustained VT (NSVT), and inducible, nonsuppressible, sustained VT/VF with programmed stimulation. Most MADIT-I patients (93%) had nonsuppressible, monomorphic VT.
The MADIT-I demonstrated a large, 54% (95% CI = 18% to 74%) relative reduction in mortality (P = .009). The MADIT-I population has been considered distinct from the patients in the AVID trial, CIDS, and CASH, but the populations are similar. Both CIDS and AVID included patients with VT unrelated to a cardiac arrest. CIDS also included patients with a history of syncope and a low LVEF value plus inducible VT. The average extension of life with an ICD was 10 months. This large reduction in mortality (26.2% absolute reduction over 27 months) represents an NNT of only 3 over 36 months. This translates into a modest incremental cost of $27,000 per life-year gained, making ICD therapy in this population very economically attractive.106
The annual mortality rate in the MADIT-I control group appears to be somewhat higher than that observed in the three trials evaluating ICD therapy in patients with mostly spontaneous sustained ventricular arrhythmias (see Table 11-2). The likely explanation is that all the patients in MADIT-I had LVEF ≤0.35, whereas LVEF was not a primary inclusion criterion in AVID, CIDS, or CASH. If only those patients in AVID, CIDS, and CASH with LVEF ≤0.35 are studied, a similar 16% annual mortality rate is observed in patients receiving amiodarone.22 Moreover, the relative reduction in risk of death with an ICD versus amiodarone among patients with LVEF ≤0.35 in AVID, CIDS, and CASH (44%; 95% CI = 17% to 47%; P <.001) is similar to that found in MADIT-I (54%; 95% CI = 18% to 74% reduction). Thus, patients with spontaneous or inducible sustained ventricular arrhythmias have a similar risk of death and benefit to a similar extent with ICD therapy.
A major limitation of MADIT-I is the lack of information about how many patients with ischemic LV dysfunction and NSVT would have to be screened to identify one patient fulfilling all the inclusion criteria for this trial. This number is estimated to be large because these criteria are present in very few (<2%) patients after MI.107 Also, targeting ICD therapy to only those at highest risk apparently has a marginal effect on reducing the burden of sudden death (see Fig. 11-2). Another concern regarding MADIT-I is the non-protocol-driven use of antiarrhythmic drugs. For example, 23% of patients assigned to conventional therapy were not receiving antiarrhythmic drug therapy at last follow-up, and only 55% of patients were receiving amiodarone at that time. The significance of this issue is questionable, given the results of SCD-HeFT (see later), which found no significant benefit or harm from amiodarone.
Multicenter Unsustained Tachycardia Trial
The randomized Multicenter Unsustained Tachycardia Trial (MUSTT) compared antiarrhythmic therapy with best medical therapy in 704 patients with coronary artery disease, LVEF ≤ 0.40, NSVT, and inducible sustained VT/VF during programmed electrical stimulation.91 It is important to emphasize that although the use or nonuse of ICD therapy in MUSTT was not randomly assigned, the results are often considered when interpreting data from the previously discussed trials.
The prognostic significance of inducible arrhythmias during invasive electrophysiologic testing was also evaluated in MUSTT.108 During 5 years of follow-up, patients with an inducible sustained ventricular arrhythmia had a significantly higher risk of cardiac arrest or death caused by arrhythmia (32%) than patients in whom a sustained arrhythmia was not inducible (24%; P <.001). Overall mortality was also statistically higher in patients in whom a sustained arrhythmia was inducible (48% vs. 44%; P = .005). However, the absolute difference in mortality (4% over 5 years) was small and of questionable clinical significance. Also, LVEF alone was unreliable in predicting long-term outcome.109 Baseline clinical variables (functional class, NSVT, age, QRS duration, inducible VT/VF, and atrial fibrillation) were found to better estimate the risks of arrhythmic death and total mortality than LVEF ≤0.30 versus >0.30.
Patients with Heart Failure or Left Ventricular Dysfunction Alone
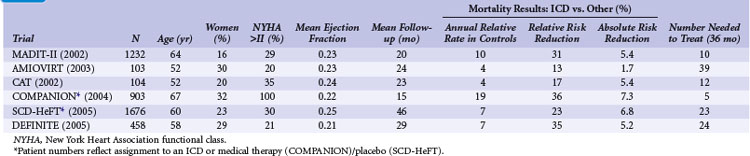
Table 11-3 summarizes the inclusion criteria, primary endpoints, and main results for six studies that involved patients with heart failure or LV dysfunction alone. Table 11-4 presents details of the study populations, the risk of the population untreated (annual control group mortality), and the relative and absolute alterations in mortality with ICD therapy. These trials are often dichotomized on the basis of the underlying etiology of LV dysfunction (ischemic vs. nonischemic). Three trials (CAT, AMIOVIRT, DEFINITE) included only patients with nonischemic LV dysfunction; two (COMPANION, SCD-HeFT) included patients with both ischemic and nonischemic etiologies, and one (MADIT-II) was limited to patients with ischemic LV dysfunction. It is important to recognize that number of patients, duration of follow-up, and resultant statistical power of these trials differ greatly. COMPANION was also different from the other trials in that all patients were required to have a QRS duration of greater than 120 msec and symptoms of advanced heart failure. COMPANION also assessed the combined use of CRT and ICD, whereas the other trials evaluated non-CRT ICD therapy. The two larger and long-term trials that used non-CRT ICD therapy, SCD-HeFT and MADIT-II, provide the most reliable information on the efficacy of ICD therapy in patients with heart failure or LV dysfunction alone.
TABLE 11-3 Clinical Trials of ICD Therapy for Heart Failure or Left Ventricular Dysfunction Alone: Inclusion Criteria, Comparison Groups, and Main Results
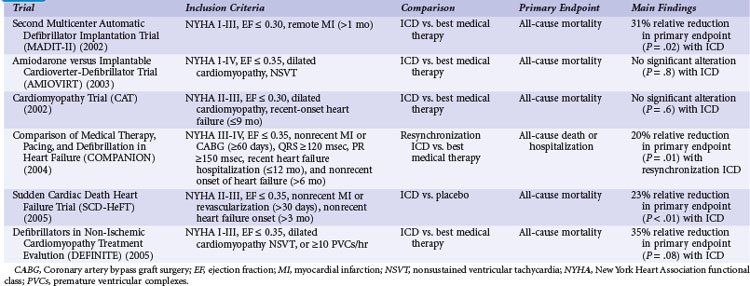
TABLE 11-4 Clinical Trials of ICD Therapy for Heart Failure or Left Ventricular Dysfunction Alone: Population Details and Mortality Results
Second Multicenter Automatic Defibrillator Implantation Trial
Although MADIT-I showed a large benefit from ICD therapy, its study population represented a highly select group of patients (see Figs. 11-2 and 11-3). MADIT-II evaluated ICD therapy in a lower-risk group that accounts for a larger proportion of patients who will experience sudden death. MADIT-II enrolled 1232 patients from 71 U.S. centers and five European centers from July 1997 to January 2002.56 ICD therapy was compared with usual care in patients with ischemic LV dysfunction (LVEF ≤0.30). Patients who had experienced a recent MI (within 1 month of evaluation) or revascularization procedure (within 3 months of evaluation) were not eligible. Patients who underwent invasive electrophysiologic testing and had inducible sustained arrhythmias, fulfilling the criteria for MADIT-I, were also ineligible. Angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and lipid-lowering therapies were prescribed to 70%, 70%, and 66% of participants, respectively, on the basis of data from the last follow-up visit. These rates are higher than in the trials evaluating ICD efficacy in patients with spontaneous or inducible ventricular arrhythmias conducted before the wider recognition of the importance of these medications.
The cost-effectiveness of ICD therapy in MADIT-II was assessed using data from 1095 U.S. patients randomized to an ICD or conventional care. Utilization data were converted to costs using national and hospital-specific data. The incremental cost-effectiveness ratio was calculated as the difference in discounted costs divided by the difference in discounted life expectancy within 3.5 years. Secondary analyses included projections of survival (using three alternative assumptions), corresponding cost assumptions, and the resulting cost-effectiveness ratios out to 12 years after randomization. Assumptions included the cost of ICD therapy was $33,000 (device and implant) and a survival rate of ICD recipients ranging from 13% to 26% at 12 years.110 The actual long-term survival of ICD recipients in MADIT-II is in keeping with the more optimistic projection.50 This survival estimate results in an incremental cost-effectiveness ratio in the range of $50,000. This result is similar to that estimated for other trials when results were extrapolated over time.57 These data indicate that ICD therapy is economically beneficial in these patients.
The MADIT-II researchers noted that the effect of ICD therapy was similar in subgroup analyses stratified by age, gender, LVEF, and NYHA class. However, patients with longer QRS durations (>120 msec) had larger absolute and relative risk reductions that trended toward statistical significance. Some considered this finding to indicate that patients with QRS values greater than 120 msec benefit from ICD therapy and that those with shorter QRS values do not, but this remains controversial.111 Delayed ventricular conduction has also been shown to predict a higher risk of death among patients receiving contemporary medical therapy.80 Other noninvasive risk assessment tools, such as T-wave alternans, have been advocated for selecting patients for ICD therapy,112 but their usefulness remains unproved83 (see Ongoing Trials).
The ability of electrophysiologic testing to predict mortality and ICD efficacy was also assessed in MADIT-II.68,113 Electrophysiologic inducibility was performed on 593 patients randomly assigned to ICD therapy. A sustained ventricular arrhythmia was inducible in 36% of patients, who were more likely to demonstrate spontaneous VT in follow-up. In contrast, patients who did not have a sustained inducible ventricular arrhythmia were more likely to experience spontaneous VF. Overall, patients in MADIT-II had a 20% rate of appropriate ICD therapies for VT or VF over 4 years of follow-up. The likelihood of VT or VF was similar for patients with and without inducible arrhythmias at baseline. As with the findings in MUSTT, the MADIT-II data indicate that electrophysiologic testing is suboptimal in identifying patients who will benefit from an ICD.
Analyses from MADIT-II include clinical risk factors associated with benefit from ICD therapy114 and with mortality in ICD recipients115 and utility of noninvasive tests to predict ICD benefit. Although both dynamic alterations in QT duration116 and morphology117 predicted an increased risk of death and arrhythmias in MADIT-II, neither measure was useful in discriminating between patients likely versus unlikely to benefit from an ICD.
A risk score comprised of five clinical factors (NYHA Class >II, age >70, BUN >26 mg/dL, QRS duration >120 msec, atrial fibrillation) was derived after excluding patients with blood urea nitrogen (BUN) values of at least 50 mg/dL and/or serum creatinine values of at least 2.5 mg/dL. Conventional therapy patients with none of these risk factors had an 8% mortality rate, and those with at least one risk factor had a 28% mortality rate. ICD therapy was not associated with a significant alteration in mortality among the 345 patients with no risk factors (hazard ratio 0.96; P = .91), but was associated with a 49% relative reduction in the risk of death in the 786 patients with at least one risk factor (P <.001). This score is interesting in that it is similar to that proposed in CIDS103 and contains some of the same variables that in MUSTT predicted the long-term risks of arrhythmic death and total mortality.109 The utility of the MADIT-II risk score is unclear because it has not been validated, and its components largely overlap with those shown to predict death despite ICD use in MADIT-II participants.115
Another interesting analysis in MADIT-II evaluated the efficacy of ICD therapy based on the time from MI to study enrollment.118 An inclusion criterion for MADIT-II was that patients had their MI at least 1 month before evaluation; however, the average time from MI to enrollment in MADIT-II was much longer (mean, 81 months). Patients were subdivided into quartiles of time from MI to enrollment: less than18 months (300 patients), 18 to 59 months (283), 60 to 119 months (284), and 120 months or longer (292). A survival benefit from ICD therapy was apparent in the three groups with a longer duration between MI and enrollment (relative risk reductions of 38% to 50% with ICD therapy), but no benefit from ICD therapy was evident among patients whose MI events had occurred within 18 months of enrollment (2% reduction). These data, along with those of DINAMIT and IRIS, suggest that ICD therapy may not be effective early after MI. This may relate to the mechanisms of sudden death early after MI,11 a differential risk of sudden versus nonsudden death early after MI, or other factors.119
Amiodarone versus Implantable Cardioverter-Defibrillator Trial
The Amiodarone versus Implantable Cardioverter-Defibrillator Trial (AMIOVIRT) compared ICD therapy versus amiodarone in patients with nonischemic LV dysfunction and asymptomatic nonsustained ventricular tachycardia (NSVT) at 10 U.S. centers from 1996 and 2000.120 Patients were required to have a nonrecent (at least 6 months) diagnosis of LV dysfunction. The primary endpoint was total mortality. A total of 103 patients were enrolled, and average follow-up was 2 years. Of 13 deaths, six were in the ICD group and seven in the amiodarone group (P = .8). AMIOVIRT was terminated early because of futility. The trial was powered to detect a 50% relative (10% absolute) reduction in mortality with an ICD versus amiodarone. The observed mortality rate in amiodarone-treated patients was 12% at 3 years. AMIOVIRT neither supports nor refutes the value of ICD therapy in patients with nonischemic LV dysfunction and NSVT.
Cardiomyopathy Trial
The Cardiomyopathy Trial (CAT), conducted in 15 German centers from 1991 to 1997, compared ICD therapy with standard medical therapy in 104 patients with nonischemic LV dysfunction.121 Unlike the other trials of ICD therapy in patients with heart failure or LV dysfunction alone, patients in CAT were required to have recently diagnosed heart failure (<9 months before enrollment). The primary endpoint of CAT was all-cause mortality. Only 30 deaths were observed over a mean follow-up of 5.5 years. Of these, 13 occurred in the ICD group and 17 in the control group (P
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree