12 Clinical Trials of Cardiac Resynchronization Therapy
Pacemakers and Defibrillators
Treatment of the heart failure disease substrate with device-based therapy such as CRT has expanded the potential of a heart failure device that not only treats heart failure, but also diagnoses and prevents heart failure exacerbations and arrhythmic events, aiding overall patient management and tracking. Patients with advanced heart failure can receive contemporary medical therapy combined appropriately with electrical resynchronization. Also, their outcome may be predicted by powerful point-of-source data sets that allow for daily diagnostic measures and patient participation in disease management.1–10
Resynchronization devices now represent about 40% of all implantable cardioverter-defibrillators (ICDs) used in the United States.11 Review of clinical trial data shows that at least one third of patients for whom ICDs are indicated also qualify for a CRT with defibrillator (CRT-D) device.12,13 This proportion is likely to increase with expansion of CRT therapy to patients with mild to moderate heart failure.14 Although the implant procedure for a CRT-D is technically more challenging than that for an ICD, CRT has the added advantages of making patients feel better, causing reverse ventricular remodeling, and reducing heart failure hospitalizations—three important goals when offering device therapy to a population with heart failure.2,9,15,16 Despite the evidence base and guidelines, CRT is underutilized in eligible patients, with significant variation in age, gender, QRS duration, care provider, insurance status, and geographic location of practices.17
This chapter also reviews advances in lead technology, and device features for delivering CRT itself or expanding CRT, such as remote device follow-up and enhanced CRT–heart failure diagnostic devices.18–23
 Heart Failure and QRS Delay: Scope of the Problem
Heart Failure and QRS Delay: Scope of the Problem
The greatest expense for the U.S. Medicare Trust Fund is the treatment of heart failure.24–28 The majority of this expense is in the acute management of heart failure hospitalizations, which often require intensive care unit management.26 There are not only about 1 million heart failure hospitalizations yearly, but also 300,000 heart failure deaths. These deaths are primarily caused by progressive pump dysfunction and sudden cardiac death, both of which can be addressed with a CRT and pacemaker (CRT-P) or CRT-D device.8,9 As heart failure severity increases, characterized by both mechanical and electrical remodeling, the primary cause of cardiovascular death typically is pump failure. Conversely, as heart failure functional class improves, electrical instability plays a more prominent role, such that the absolute number of sudden deaths in the patient with advanced heart failure and QRS prolongation is significant, accounting for about one third of all deaths.29,30
When it accompanies heart failure from systolic dysfunction, QRS delay (≥120 msec) itself adds significant morbidity and mortality.31–36 In fact, mortality rates progressively increase as intraventricular conduction delay increases. The latter may also predispose heart failure patients to an increased risk of ventricular arrhythmias by acting as a substrate for reentrant ventricular tachycardia.37
Affecting 30% to 50% of patients with New York Heart Association (NYHA) Class III or IV heart failure, QRS delay, predominantly left bundle branch block (LBBB), impairs cardiac function by introducing intraventricular dyssynchrony. Severe mechanical left ventricular (LV) dyssynchrony is observed in 60% to 70% of patients with QRS duration of 120 msec or greater. It was previously thought that this group of patients with advanced heart failure would be most likely to benefit from resynchronization therapy; this chapter reviews data disproving this assumption. Conduction delay also worsens atrioventricular (AV) and interventricular (VV) dyssynchrony, whereas intraventricular dyssynchrony results in worsening LV function, as measured by the rise of left ventricular pressure (dP/dt) and filling times.38–41 Interestingly, even in patients with right bundle branch block (RBBB) or intraventricular conduction delay, significant electrical delay to the left ventricle is observed on detailed activation mapping, suggesting that RBBB in this setting often represents “concealed” LBBB.42,43
 Studies of CRT in the Acute Setting: How Does It Work?
Studies of CRT in the Acute Setting: How Does It Work?
Cardiac resynchronization therapy is defined as the stimulation of the left ventricle or simultaneous stimulation of both the right and the left ventricle after atrial sensed or paced events or in atrial fibrillation (AF). CRT works by multiple mechanisms, ranging from structural changes (e.g., favorable ventricular remodeling, improved peak oxygen consumption, reduced mitral regurgitation) to cellular and molecular changes (e.g., improved adrenergic-stimulated myocyte function, decreased neurohormonal activation, altered ionic currents and calcium homeostasis). In an elegant study, Tomaselli’s group recently demonstrated that CRT abbreviates the dyssynchronous heart failure–induced prolongation of action potential in cells isolated from the LV lateral wall. Aiba et al.44 conclude that CRT partially reverses the cellular triggers and substrate for arrhythmias in this pacing-induced model of heart failure. CRT works by partially or totally correcting AV, VV, and most importantly left intraventricular dyssynchronies, leading to reverse remodeling and restoring adverse electrophysiologic, neurohormonal, and anatomic changes that result from heart failure.40,44–50 The predominant beneficial effects are on measures of systolic function, as summarized in Table 12-1 and discussed in detail in Chapter 9.
TABLE 12-1 Mechanisms of Acute Improvement in Cardiac Function with Cardiac Resynchronization Therapy (CRT)
| Type of CRT | Mechanisms |
|---|---|
| Atrioventricular (AV) resynchronization | Diminished mitral regurgitation |
| Lengthened diastolic filling time | |
| Optimization of filling pattern | |
| Inter/intraventricular resynchronization | Increases in left ventricular efficiency/systolic blood pressure, dP/dt, pulse pressure, stroke volume, and stroke work |
| Decrease in left ventricular end-systolic volume |
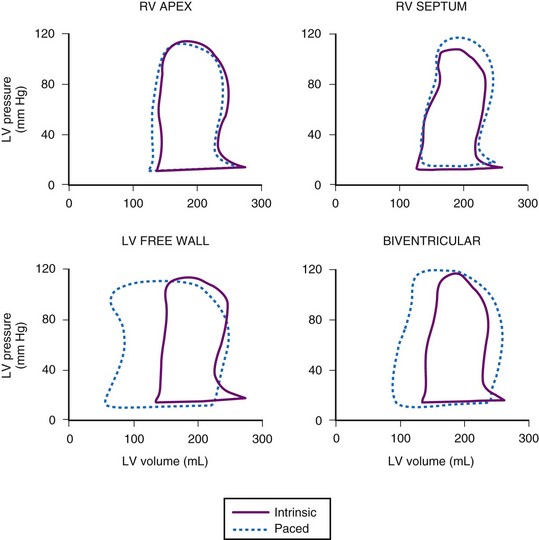
In the first closed-chest study of CRT, in 27 subjects with heart failure and QRS delay, Blanc et al.45 demonstrated improvements in systolic blood pressure, pulmonary capillary wedge pressure, and V-wave amplitude with LV or biventricular (BiV) stimulation compared with baseline or right ventricular (RV) pacing. Kass et al.39 and Aurrichio et al.46 subsequently demonstrated that the effects of CRT could be further optimized by AV delay timing to achieve immediate increases in dP/dt and pulse pressure of 12% to 25% with LV or BiV stimulation, in a total of 45 patients. Figure 12-1 demonstrates the effects of pacing site on pressure-volume loops obtained in a patient with heart failure and LBBB. Neither RV pacing site alters the abnormal loop. However, both left ventricular free wall (LVFW) and BiV stimulation result in reduced LV end-systolic volume and increased stroke volume and stroke work (increased loop width and area). These changes correlated with improved pulse pressure. Interestingly, in the patient with AF and heart block, greater immediate improvement in LV function is achieved with BiV or LV-RV offset stimulation than with single-site LV stimulation, presumably because VV dyssynchrony induced by LV-only stimulation is avoided in the setting of heart block.49
Acute predictors of a beneficial hemodynamic response to CRT were identified to be baseline extent of QRS delay (but not subsequent shortening with pacing) and mechanical dyssynchrony.41,50 LVFW rather than true anterior LV stimulation sites appear to elicit a more robust acute hemodynamic response.48 However, rigorous endocardial mapping has recently shown a high degree of individual variability for the best pacing site.51 The authors concluded that in a homogeneous group of patients with nonischemic dilated cardiomyopathy, the optimal pacing site cannot be predicted, and pacing from the coronary sinus or midlateral wall is rarely optimal.
Speckle tracking analysis of torsion suggests that an acute improvement in LV twist after CRT predicts LV reverse remodeling.52 Similar findings in the PROMISE-CRT study support the hypothesis that acute changes in radial mechanical dyssynchrony are associated with LV reverse remodeling.53
 Controlled Trials of CRT Devices
Controlled Trials of CRT Devices
Table 12-2 summarizes the design, inclusion criteria, and results of the early controlled clinical trials of CRT-P and CRT-D devices. In general, inclusion criteria were similar: symptomatic, mostly NYHA Class III and IV heart failure (except REVERSE and MADIT-CRT, later trials that enrolled NYHA I and II patients), left ventricular ejection fraction (LVEF) of less than 0.35, prolonged QRS duration (>120, >130, or >150 msec), and stability of proved medical therapies for heart failure before enrollment.3,5,7–10,54–57
TABLE 12-2 Early Controlled Trials of CRT Alone or with Implantable Cardioverter-Defibrillator (ICD)
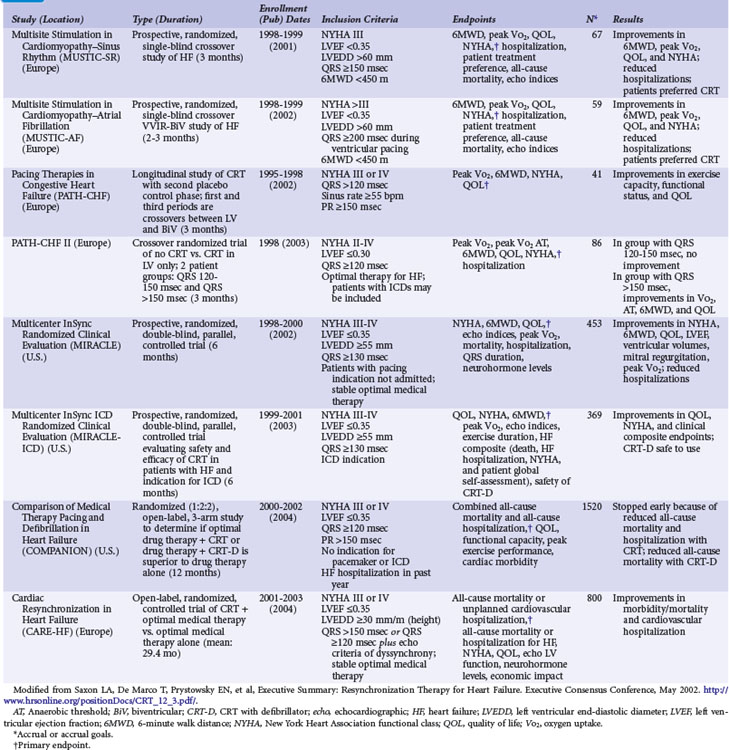
Only two early trials used epicardial LV leads, placed through limited thoracotomy for LV stimulation.4,54 The Multisite Stimulation in Cardiomyopathy–Sinus Rhythm (MUSTIC-SR) trial used a stylet-driven coronary sinus lead; the other trials used over-the-wire leads to achieve LV stimulation through a coronary sinus branch vein.3,7–10,54–57 The earliest U.S. CRT study using an epicardial LV lead, the VIGOR-CHF trial, was not completed because of insufficient patient enrollment for the primary functional endpoint of peak oxygen uptake (peak Vo2). In addition, with the emergence of the coronary sinus branch vein lead, patients and physicians became reluctant to continue using a more invasive procedure for LV stimulation. The echocardiographic substudy, however, as with the first European Pacing Therapies in Congestive Heart Failure (PATH-I) study, demonstrated improvement in several measures of response to CRT, comparable to levels seen in studies using transvenous LV stimulation for CRT.7,40,54 The VIGOR-CHF trial demonstrated a decrease in LV and left atrial volumes as well as improvements in LV outflow tract and aortic velocity time integrals and myocardial performance indices with just 12 weeks of CRT. The severity or grade of mitral regurgitation as well as mitral deceleration (a measure of improvement of diastolic LV function) also improved.
In the two early U.S. trials that were the basis for attaining the initial approvals from the U.S. Food and Drug Administration (FDA) for CRT-defibrillator (CONTAK CD and MIRACLE-ICD; see later), patients with NYHA Class II were included, but FDA labeling was not requested for this patient subset and was granted only for patients with NYHA III and IV.8,57,58 Exclusion criteria included the presence of an implanted device and requirement for bradycardia pacing support or permanent AF. The early U.S. trials used parallel design; devices were implanted in all patients, who were then randomly assigned to “CRT on” or “CRT off” status for 6 months. Two principal investigators at each enrolling center were designated in most trials, so the physician managing the medical therapies (heart failure) was blinded as to the treatment assignment, and the implanting physician (electrophysiologist) followed the device performance.
The study endpoints in CRT trials have evolved over time. Although all trials have included safety and efficacy endpoints, the initial trials assessed only measures of heart failure functional status, LV systolic function (LVEF), and LV remodeling (LV end-systolic and diastolic dimensions). The later, larger studies targeted mortality and hospitalization endpoints (Table 12-3). The use of these multiple endpoint measures is standard for heart failure trials evaluating medical therapies and has highlighted the issue of defining “benefit” from CRT.1,6 One can define “response” as consisting only of symptom improvement, or one can require that all three measures of heart failure show benefit, as outlined in Table 12-3. To complicate the issue further, no 1 : 1 correlation seems to exist between these measures of response. Again, the CONTAK CD and MIRACLE-ICD studies enrolled some patients with NYHA Class II in addition to those with NYHA III and IV functional status. In the NYHA II group, significant improvements in measures of functional status were not uniformly observed, although some patients experienced a positive reverse-remodeling response.57,58 This has been confirmed in the much larger REVERSE study, where CRT in patients with NYHA Class I/II heart failure resulted in major structural and functional reverse remodeling at 1 year, with the greatest changes in patients with nonischemic cardiomyopathy.15 Clearly, CRT has a positive effect on decreasing LV size, and the subsequent MADIT-CRT study demonstrated that CRT favorably alters the natural history of heart failure by reducing hospitalization.14,59
TABLE 12-3 Study Endpoints in Trials of CRT Devices
| Measure | Endpoints |
|---|---|
| Functional status | |
| Heart failure progression | |
| Heart failure outcome |
Multisite Stimulation in Cardiomyopathy Studies
The 2001-2002 European Multisite Stimulation in Cardiomyopathy (MUSTIC) studies provided the first long-term controlled trial data on the efficacy of CRT, delivered as BiV stimulation, for 3-month intervals, compared with normal sinus rhythm or continuous RV-based pacing in AF patients.3,56 Figure 12-2 illustrates the crossover study design of the MUSTIC and MUSTIC–Atrial Fibrillation studies. Although only 48 patients completed the two 3-month crossover study periods, all leads placed in the trial were transvenous, with no significant safety issues. Eligibility for patient enrollment included NYHA Class III with QRS longer than 150 msec. In patients with normal sinus rhythm, quality of life (QOL) score improved by 32%, 6-minute walk distance (6MWD) improved by 23%, and peak Vo2 improved by 8%. Although the study was not statistically powered to determine a reduction in rate of hospitalization, hospitalizations after CRT initiation decreased by two thirds.3 At the end of the crossover phase, patients (who were blinded to treatment) were asked to choose which 3-month period they preferred; 85% chose the pacing period during which they had been assigned to VDD, 10% had no preference, and 4% chose ODO (no pacing). Four patients had severe episodes of congestive heart failure exacerbation during the ODO pacing period.
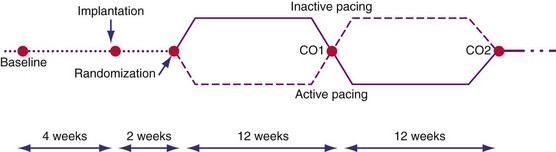
Figure 12-2 Crossover study design of MUSTIC studies.
(From Cazeau S, Leclercq C, Lavergne T, et al: Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 344:873-880, 2001.)
In the patients with permanent AF and continuous RV pacing, 37 of 59 who underwent CRT implantation completed both 3-month crossover phases and had documentation of 97% to 100% CRT delivery. Because of the significant number of dropouts (42%), the intention-to-treat analysis did not show a significant improvement with CRT. In the 37 patients with a complete data set and documentation of CRT, QOL measure did not improve, but 6MWD and peak Vo2 increased significantly, by 9% (P = .05) and 13% (P = .04), respectively.56 When the entire 6-month crossover phase is considered, 10 of 44 patients were hospitalized for heart failure decompensation during RV pacing, whereas only three were hospitalized for heart failure during the CRT period; 85% of patients preferred the CRT period. There was a trend toward a better QOL among patients with CRT (11% improvement; P = .09). Subsequent uncontrolled trials in patients with permanent AF and continuous RV pacing have shown a more robust improvement in these measures, as well as a reverse-remodeling response with CRT compared with RV pacing alone.60,61 The PAVE trial also showed improvement with CRT but did not require heart failure caused by systolic dysfunction for enrollment.62
Pacing Therapies in Congestive Heart Failure
The two Pacing Therapies in Congestive Heart Failure (PATH-I and PATH-II) European studies were groundbreaking in that chronic device programming was based on acute hemodynamic measures of cardiac performance. In addition, these same measures were used to optimize AV-delay programming.54,55 Begun in 1995 and completed in 1998, these trials enrolled patients with NYHA Class III or IV congestive heart failure, sinus rate higher than 55 beats per minute (bpm), and QRS duration longer than 120 msec.
In PATH-I, patients were crossed-over between LV and BiV stimulation with a 1-month interval of no stimulation. A second study phase lasted 9 months and used the CRT mode that achieved what the follow-up physician determined was the most optimal mode. There were no differences in the acute or chronic response of patients whether programmed to LV or BiV CRT. Statistically significant improvements in peak Vo2 anaerobic threshold (24% improvement; P < = .001), 6MWD (25%; P < .001) and QOL (59%; P < .001) were observed at 3 months and 12 months of follow-up. Of 29 patients followed to 12 months, 21 improved from NYHA Class III or IV to Class I or II. Heart failure hospitalizations decreased from 76% in the year before implantation to 31% during the year after implantation. Importantly, LBBB was the type of conduction delay in more than 87% of patients; most CRT trials enroll up to 30% of patients with either intraventricular condition delay (IVCD) or RBBB.4–69 This difference may explain why LV stimulation in PATH-I resulted in only an equivalent response to BiV stimulation to achieve CRT, although the study was not statistically powered to demonstrate a difference between the two modalities, and the long-term data were pooled from both modes. The best that one can conclude is that in small numbers of patients who undergo hemodynamic optimization programming during implantation that shows equivalence between LV and BiV stimulation to achieve CRT, long-term symptom responses appear to be equivalent.
Extending the observations from PATH-I, PATH-II evaluated LV-only CRT compared with no CRT in a 3-month crossover design.55 In all patients with LBBB (88% of subjects), LV pacing was identified as the optimal single-chamber pacing mode (compared with RV only) on the basis of immediate hemodynamic response, and AV delay timing was optimized in all patients. Patients were further divided by QRS duration according to whether the QRS was more than 120 msec but less than 150 msec (“short QRS”) or more than 150 msec (“long QRS”). Unfortunately, only 35 patients, slightly less than one half of all patients enrolled, completed both 3-month crossover intervals. Nonetheless, the study did demonstrate improvements in peak Vo2, anaerobic threshold, 6MWD, and QOL in the patients with long QRS. For example, 71% of the long-QRS group and 38% of the short-QRS group had an increase in the peak Vo2 of more than 1 mL/kg/min with active pacing. This was the first study to demonstrate that QRS duration predicts the magnitude of symptom response to CRT delivered as LV-only stimulation. Subgroup analysis of all but one of the larger U.S. long-term studies of BiV CRT also suggests that the magnitude of benefit may be greater in patients with longer QRS duration at baseline.5,8–10,57 The EARTH trial will compare chronic LV to BiV stimulation and RV stimulation and assess differences in symptoms and in echocardiographic and metabolic exercise test performance, according to stimulation mode in CRT candidates.63
Multicenter InSync Randomized Clinical Evaluation
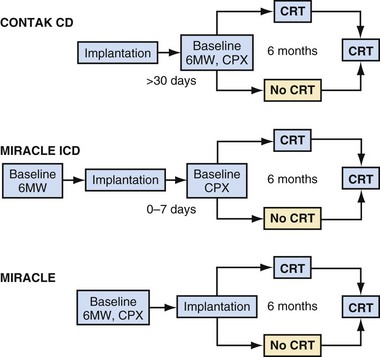
The Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study was the only U.S. trial of CRT for heart failure that used a CRT-pacemaker device only.5 The number of patients randomly allocated in U.S. clinical trials was much greater than those in the European trials until the CARE-HF trial. All 453 patients enrolled in the MIRACLE study underwent implantation of the CRT device and then random assignment to “CRT on” or “CRT off” status for 6 months. Figure 12-3 illustrates the study design of the MIRACLE, MIRACLE-ICD, and CONTAK CD U.S. trials. Unlike the COMPANION trial, in which patients were randomly assigned after consent was obtained and before device implantation, these earlier U.S. trials randomly assigned patients only after a successful CRT implant. The U.S. trials also employed strict protocol-mandated criteria on appropriate and stable heart failure medical regimen requirements before consent and device implantation.
The primary endpoints, including 6MWD, QOL score, and NYHA functional class, were all favorably influenced by CRT, and the effects of CRT were apparent as early as 1 month after therapy initiation. Patients who underwent CRT showed 13% improvement in 6MWD, 13% improvement in QOL, about 1-mL/kg/min improvement in exercise capacity, and an increase in total exercise time of approximately 60 seconds. Unlike the European and acute hemodynamic studies, neither baseline QRS duration nor type of bundle branch block influenced response to CRT in the MIRACLE study. The secondary endpoints, Vo2 and LVEF, also improved with CRT, as did episodes of heart failure worsening, including heart failure hospitalizations. At 6 months, CRT was associated with decreased LV end-diastolic volume (LVEDV) and LV end-systolic volume, reduced LV mass, increased LVEF (+3.6%), decreased mitral regurgitation jet area (−2.5 cm2), and improvement in the clinical composite heart failure score. Improvements in LVEDV and LVEF were twofold greater in patients with nonischemic cardiomyopathy. CRT resulted in significant improvements in NYHA class and LVEF, regardless of age.64
CONTAK CD and Multicenter InSync ICD Randomized Clinical Evaluation
Concurrent with the MIRACLE trial enrollment, two large-scale trials of CRT-defibrillator for patients with heart failure and primary or secondary indications for an ICD were also enrolling subjects, the Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE-ICD) and the CONTAK CD Biventricular Pacing Study. Unlike the MIRACLE study, the 950 patients randomly assigned to different therapies in the CRT-D studies had primarily ischemic cardiomyopathy (61%-75%,) and about one half of the patients had a secondary indication for the ICD.8,57 In patients with NYHA Class III or IV status, both studies demonstrated improvements in functional measures of heart failure status. An ongoing debate concerns the impact of CRT and reverse remodeling on ventricular arrhythmias. Neither study showed a difference in the incidence of treated episodes of ventricular tachycardia or ventricular fibrillation (VT/VF) with CRT on or off, indicating a neutral effect of CRT on the arrhythmia substrate early after device implantation. A subsequent analysis of the MIRACLE-ICD data indicated that patients with secondary ICD indications experienced more ICD therapies for VT, whereas those with primary ICD indications had more therapy for VF.65 The incidence of ICD therapy, as expected, was higher in those with secondary prevention indications. In CONTAK CD, the incidence of ICD therapy over the 6-month follow-up was 16% for both VT and VF. In contrast, in the InSync ICD Italian Registry, a significant reduction in ventricular arrhythmias and shock therapies was reported and correlated with the degree of ventricular remodeling at 12 months.66 Similar findings were seen in the InSync-III Marquis study, in which anatomic responders to CRT demonstrated fewer premature ventricular contractions (PVCs), runs of PVCs, and therapies for VT/VF.67
Improvements in LVEF and ventricular size and dimension and degree of mitral regurgitation were noted in the VIGOR-CHF, MIRACLE, MIRACLE-ICD, and CONTAK CD studies, all of which had core echocardiographic laboratories performing analysis, with excellent intraobserver and interobserver variability.7,40 As mentioned, even the patients with NYHA Class II benefited from CRT in terms of an echocardiographic response of reverse remodeling.16,57 These CRT-related effects were independent of the use of β-blocker therapy.7 This finding suggests that CRT can exert beneficial effects on the remodeling process across a spectrum of heart failure severity, similar to that observed with angiotensin-converting enzyme (ACE) inhibitor therapy.1 A subsequent study of the effects of CRT on ventricular function, volume, and dimension has shown that the beneficial effects occur as early as 4 weeks and are sustained for a time even after CRT is suspended, indicating that CRT affects cardiac structure.68
Another measure of heart failure progression, level of plasma neurohormones, did not improve or worsen with CRT in the MIRACLE-ICD or MIRACLE study. This neutral effect may be a result of optimization of medical therapy with neurohormonal antagonists before device implantation or inadequate duration of follow-up.7
Comparison of Medical Therapy, Pacing, and Defibrillation on Heart Failure
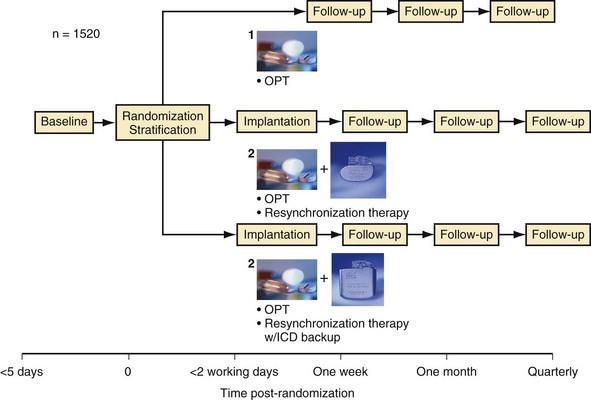
The Comparison of Medical Therapy, Pacing, and Defibrillation on Heart Failure (COMPANION) study was the first and only U.S. trial statistically powered to assess the impact of CRT on hospitalization and mortality endpoints.9,69 During COMPANION’s design, it was unclear whether ICD therapy in addition to CRT would reduce mortality in advanced heart failure compared with medical therapy. Therefore, the study randomly assigned patients to optimal medical (pharmacologic) therapy for heart failure (OPT), a CRT with pacemaker (CRT-P) alone, or a CRT with an ICD (CRT-D). The patients were assigned in a 1 : 2 : 2 ratio, respectively, to maximize the number of patients receiving devices. Statistical power was insufficient to compare CRT with CRT-D directly (both were compared with OPT), but the highest-order secondary endpoint was mortality. To enrich the anticipated event rate in the trial, patients also needed to have heart failure hospitalization in the previous year, but not in the month preceding enrollment, and to be receiving stable medical therapy at enrollment. Unlike the prior trials of CRT, patients were assigned for therapy and data were analyzed after they had provided informed consent, not after they had undergone a successful implantation.
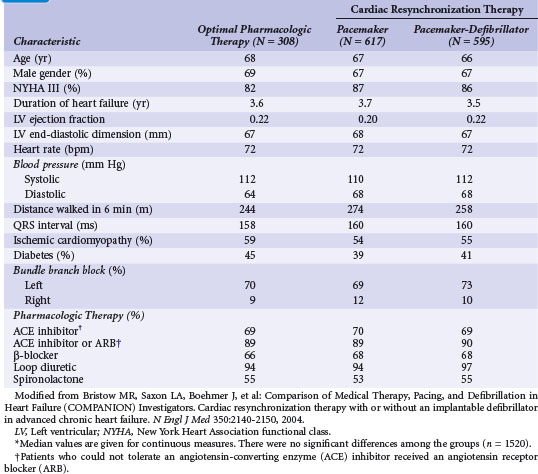
Figure 12-4 provides the design of the COMPANION study, and Table 12-4 lists the clinical characteristics of COMPANION patients. As with MIRACLE patients, and unlike those in the CRT-D studies, an equal proportion of patients in COMPANION had both ischemic and nonischemic etiologies for LV dysfunction. This was the first trial to enroll patients with advanced heart failure who were medically treated with “triple therapy,” consisting of ACE inhibitors, β-receptor blockers, and aldosterone antagonists.
The primary study endpoint was a composite of all-cause hospitalization and mortality, and the secondary endpoint was mortality (1520 patients enrolled). Figure 12-5 shows the event-free survival curves for the primary and secondary endpoints and demonstrates the 20% 12-month reduction in death or hospitalization from any cause observed with both CRT and CRT-D devices compared with OPT. The risk of one of these events was 68% in OPT patients, attesting to the severity of heart failure in this population. Although CRT-P reduced mortality by 24%, this was not statistically significant (P = .06). CRT-D alone reduced mortality by 36% (P = .003) compared with OPT. Close inspection of the mortality curves shows that CRT survival parallels OPT survival until 6 months, when CRT shows benefit. In contrast, the CRT-D and OPT curves separate immediately.
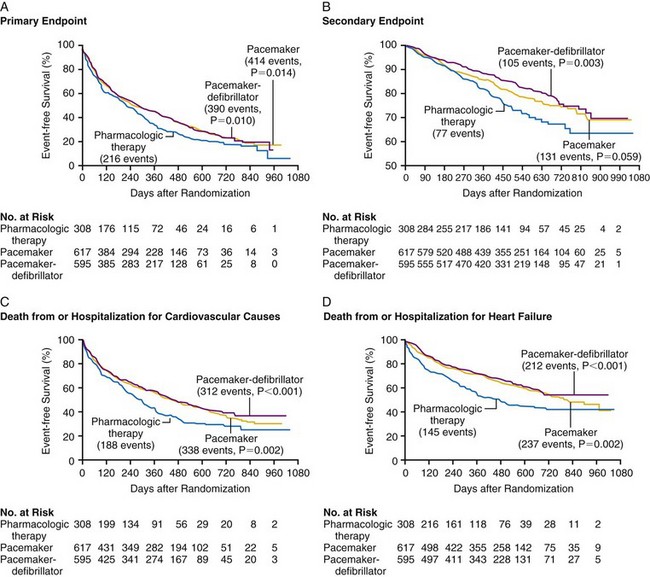
Figure 12-5 Kaplan-Meier estimates in COMPANION study.
(From Bristow MR, Saxon LA, Boehmer J, et al: Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 21;350:2140-2150, 2004.)
These observations suggest that the ICD portion of the CRT-D has an immediate effect to prevent sudden arrhythmic death, whereas the reductions in sudden death with CRT alone may be mediated through stabilization of heart failure status, which may be time dependent. Subsequent analysis showed that the reduced mortality with CRT-D resulted from a decrease in sudden cardiac death, and that the true reduction in hospitalizations resulted from a decrease in heart failure hospitalization, as adjudicated by an events committee.69,70 Subgroup analyses were remarkably consistent in demonstrating CRT benefit in all patient subgroups. There appeared to be equal benefit in women and men, in either ischemic or nonischemic etiologies of heart failure, regardless of LVEF greater or less than 20% and LV size greater or less than 67 mm. Those patients with longer QRS duration did appear to experience greater benefit with CRT, as did those with LBBB rather than RBBB or IVCD. Figure 12-6 provides the subgroup analysis from the COMPANION study, comparing CRT and CRT-D with OPT therapy for the primary and secondary endpoints.
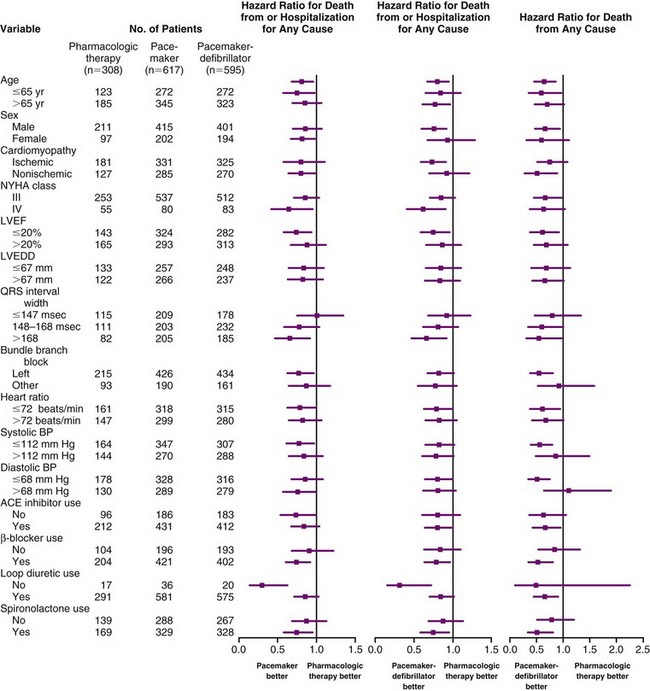
Figure 12-6 Patient characteristics and hazard ratios in COMPANION study.
(From Bristow MR, Saxon LA, Boehmer J, et al: Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators: Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350:2140-2150, 2004.)
Any concern that the implantation of a CRT device may destabilize the usually very ill NYHA Class IV patient was addressed in a post hoc analysis of COMPANION.71 Although these devices did not impact significantly on heart failure deaths, both CRT and CRT-D significantly improved time to all-cause mortality and hospitalizations in the NYHA Class IV subset of patients. Time to sudden death was significantly reduced in the CRT-D group.
The COMPANION data expand the role of CRT to achieve the three primary therapeutic goals in treating patients with heart failure: to improve symptoms, retard disease progression, and reduce rates of hospitalization and mortality. There are two primary reasons for selecting a CRT-D over CRT-P alone. The COMPANION data support early sudden death protection with CRT-D, and the majority of patients with CRT indications also have ICD indications in single-chamber, primary prevention ICD trials.13,14 The 12-month and 24-month appropriate ICD therapy rate was 12% and 19%, respectively, higher than observed in the primary prevention ICD trials. Appropriate ICD therapy was predictive of risk of subsequent hospitalization and death, suggesting that sustained ventricular arrhythmias are a harbinger of worsening heart failure in CRT-D recipients.72
Cardiac Resynchronization-Heart Failure Study
The Cardiac Resynchronization-Heart Failure (CARE-HF) study, enrolling 813 patients at 82 European centers, compared CRT only with optimal medical therapy.10 Over a mean follow-up of 29 months, CRT resulted in significant reductions in the primary composite endpoint of death or cardiovascular hospitalization. Reductions were also achieved in the secondary endpoint of mortality. Figure 12-7 illustrates the Kaplan-Meier curves for these endpoints.
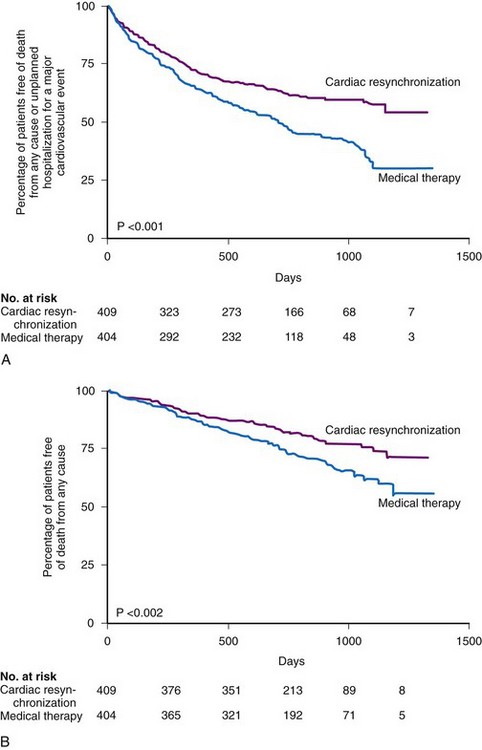
Figure 12-7 Kaplan-Meier estimates in CARE-HF study.
(From Cleland JG, Daubert JC, Erdmann E, et al: Cardiac Resynchronization-Heart Failure [CARE-HF] Study Investigators: The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539-1549, 2005.)
Comparing CARE-HF with COMPANION shows COMPANION patients to be a sicker group, perhaps because heart failure hospitalization was required in the year before enrollment.9 The 12-month mortality rate in the medical therapy group in CARE-HF was 12.6%, versus 19% in COMPANION. Only 38% of the patients in CARE-HF had coronary artery disease, compared with 56% in COMPANION; the mean LVEF was 25% in CARE-HF, compared with 21% in COMPANION; and a higher percentage of patients in COMPANION had NYHA Class IV status (16% vs. 6.5% in CARE-HF). Another issue is the positive effect of CRT on mortality in CARE-HF. The relative risk reduction with CRT in CARE-HF patients was equivalent to that of the COMPANION CRT-D patients (36%). The COMPANION CRT-P group’s 24% reduction in mortality was not statistically significant. These differences may be a result of the shorter duration of follow-up in COMPANION; with longer follow-up, the value may have become significant. Another issue was the risk of sudden death in the CARE-HF patients given CRT; although only 8% of these patients were adjudicated as dying “suddenly,” these accounted for 37% of all deaths. COMPANION data indicate such deaths may have been prevented with a CRT-D device.69
The CRT-P patients in CARE-HF had a reduced risk of sudden death at extended follow-up of 37 months, supporting the concept that chronic stabilization of heart failure status reduces arrhythmias73,74 (Fig. 12-8).
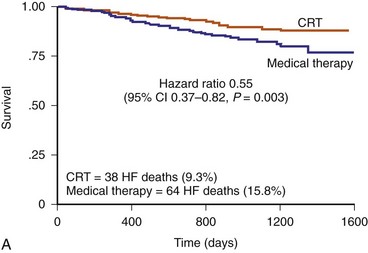
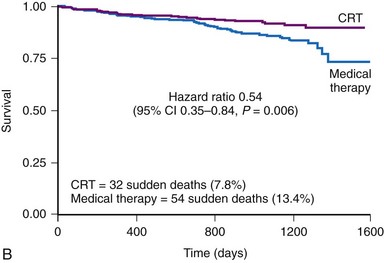
Figure 12-8 Kaplan–Meier estimates in CARE-HF extension phase.
(From Cleland JGF, Daubert JC, Erdmann E, et al: Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J 27:1928-1932, 2006.)
The CARE-HF trial answered several other important issues. CRT induces sustained LV reverse remodeling, with the most marked effects in the first 3 to 9 months and continuing up to 29 months.75 The benefits of CRT in patients with and without an ischemic etiology were similar in relative terms.76 Before CARE-HF, follow-up beyond 12 months was available to far too few patients undergoing CRT. This is the first large trial to demonstrate benefit with respect to biomarker measurements as a surrogate of congestive heart failure severity with CRT. A nonsignificant decrease in N-terminal pro–brain natriuretic peptide (NT-BNP) was noted at 3 months, but by 18 months, there was a dramatic decrease of more than 1100 pg/mL (P
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree